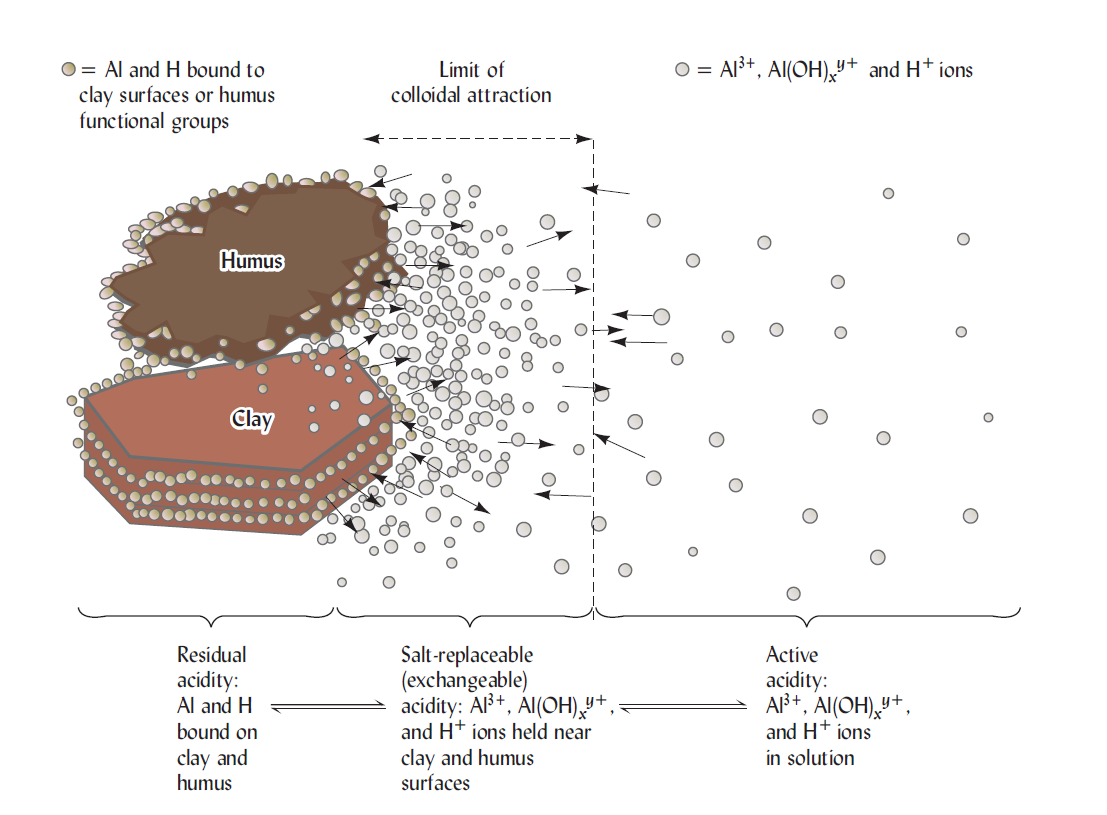
Research suggests that three major pools of acidity are common in soils: (1) active acidity due to the H+ ions in the soil solution; (2) salt-replaceable (exchangeable) acidity, involving the aluminum and hydrogen that are easily exchangeable by other cations in a simple unbuffered salt solution, such as KCI; and (3) residual acidity, which can be neutralized by limestone or other alkaline materials. But, it cannot be detected by salt replacement. These types of acidity combinedly are called the total acidity of soil. In addition, a much less common, but sometimes very important fourth pools, namely, potential acidity, can arise upon the oxidation of sulfur compounds in certain acid sulfate soils.
Active Acidity
The active acidity pool is defined by the H+ ion activity on the soil solution. This pool is very negligible compared to the acidity in the exchangeable and residual pools. Such as for neutralizing the active acidity in the upper 0.15 m of a hectare of an average mineral soil at pH 4 and 20% moisture only about 2kg of CaCO3 are required. Even so, the active acidity is extremely important, since it determines the solubility of many substances and provides the soil solution environment to which plant roots and microbes are exposed. Very acid soils contain aluminum ions in solution, which can add to the active acidity as they hydrolyze.
Best safe and secure cloud storage with password protection
Get Envato Elements, Prime Video, Hotstar and Netflix For Free
Best Money Earning Website 100$ Day
#1 Top ranking article submission website
Exchangeable (Salt-Replaceable) Acidity
Exchangeable acidity is primarily associated with exchangeable aluminum and hydrogen ions. These ions are present in large quantities in very acid soils. Cation exchange with an unbuffered salt, such as KCI can release these ions into the soil.
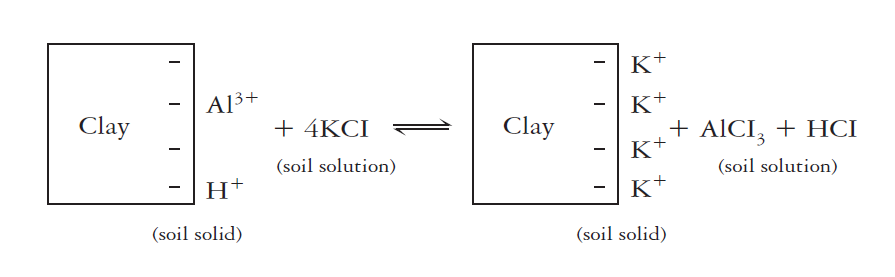
Once released to the soil solution, the aluminum hydrolyzes to form additional H+. The chemical equivalent of salt-replaceable acidity in strongly acids soils is commonly thousands of times that of active acidity in the soil solution. Even in moderately acid soils, the limestone needed to neutralize. This type of acidity is commonly more than 100 times that needed to neutralize the soil solution (active acidity). At a given pH value, exchangeable acidity is the highest soil whose clay fraction is governed by smectites, intermediate for vermiculites and lowest for kaolinite.
Residual Acidity
Together, exchangeable (salt-replaceable) and active acidity account for only a little portion of the total soil acidity. The remaining residual acidity is generally accompanied by hydrogen and aluminum ions (including the aluminum hydroxy ions). Those ions are attached in non-exchangeable forms by organic matter and clays. As the pH increases, the attached hydrogen dissociates and the attached aluminum ions are released and precipitate as amorphous AI(OH)30. These changes free up negatively charged cation exchange sites and increase the cation exchange capacity. The reaction with clays and a liming material [ e.g., Ca(OH)2] exhibits how the bound hydrogen and aluminum can be released
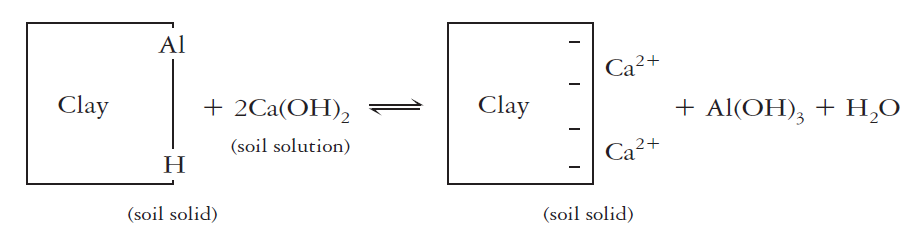
The residual acidity is commonly far larger than either the active or salt-replaceable acidity. It may be 1000 times larger than the soil solution or active acidity in sandy soil and 50,000 or even 100,000 times greater in clayey soil high in organic matter. The amount of ground limestone recommended to at least party neutralize residual acidity in the upper 15 cm of a strongly acid soil may be at least 5-10 metric tons (Mg) per hectare (2.25-4.5 tons per acre).
Total Acidity
For most soils (not potential acid sulfate soils) the total acidity that must be suppressed to raise the soil pH to the desired value. It can be defined as:
Total acidity= active acidity+ salt-replaceable acidity + residual acidity
We can conclude that the pH of the soil solution is only the tip of the iceberg in determining how much lime may be required to compensate effects of soil acidity.
References & Other Links
- The Nature and Properties of Soils Fifteenth edition by Ray R. Weil & Nyle C. Brady
 Plantlet The Blogging Platform of Department of Botany, University of Dhaka
Plantlet The Blogging Platform of Department of Botany, University of Dhaka






Very helpful. Thanks