An enormous range of plant substances are covered by the term ‘Terpenoids’ which is used to indicate that all such substances have a common origin. This Terpenoids are a diverse group of naturally occurring organic compounds mostly found in plants. They are any of a class of hydrocarbons that consist of terpenes attached to an oxygen containing group.
Terpenoids have unsaturated molecules composed of linked isoprene units generally having the formula (C5H8)n. That’s why sometimes they are called Isoprenoids.
https://area-info.net/global-isoprene-market-key-manufacturers-analysis-2017-2022/
Good to know
- Terpenes are the largest class of secondary metabolites, some are volatile and insoluble in water, biosynthesized from acetyle-CoA or glycolytic intermediate.
- Terpenoids are terpene like substances or derivatives of terpenes or modified terpenes.
Characteristics of Terpenoids
Best safe and secure cloud storage with password protection
Get Envato Elements, Prime Video, Hotstar and Netflix For Free
Best Money Earning Website 100$ Day
#1 Top ranking article submission website
- They are naturally occurring organic chemicals.
- They are the secondary metabolites or secondary plant products.
- They are generally lipophilic substances.
- They constitute the largest group of secondary plant products and show some of the properties of lipid.
- They are insoluble in water.
- They derived from a simple 5-C unit, isoprene which has a branched carbon skeleton. Isoprene inturn is derived from basic 5-C unit called as isoprene.
- Family includes hormone (Gibberellin and Abscisic acid), the carotenoid pigment (Carotene and Xanthophylls), steroids (Ergosterol, Sitosterol, Cholesterol and steral derivatives); Latex and many of the essential oils that give plant their distinctive odors and flavors.
- They are located in the cytoplasm of plant cell.
Classification of Terpenoids
Terpenoids range from the essential oil components the volatile monoterpenes and sequiterpenes(C10 and C15) through less volatile diterpenes (C20) to in volatile triterpenoids and sterols(C30) and carotenoid pigments (C40). Terpenoids are classified according to number of isoprene units used –
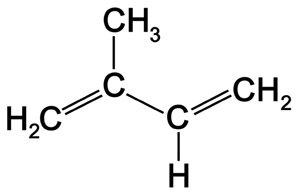
1. Hemiterpenoids
- 1 isoprene unit present (5 carbon)
- e.g. Prenal (Sweet fruit), Isovaleric Acid (Dizzy odor)
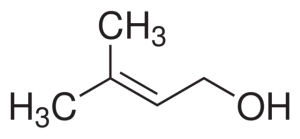
Prenol structure
https://www.addexbio.com/productdetail?pid=3113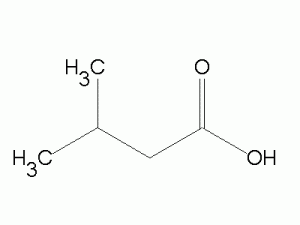
Isovaleric Acid
http://www.rdchemicals.com/chemicals.php?mode=details&mol_id=7968
2. Monoterpenoids
- 2 isoprene unit present (10 Carbon).
- e.g. Geraniol (Rose like odor) , Limnene (lemon)
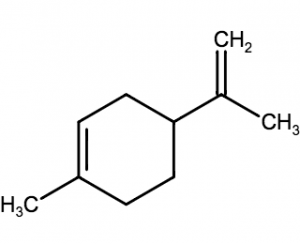
Limanene
https://www.chegg.com/homework-help/questions-and-answers/limonene
3. Sequiterpenoids
- 3 isoprene unit present (15 Carbon).
- e.g. Humelene (Sweet flower odor)
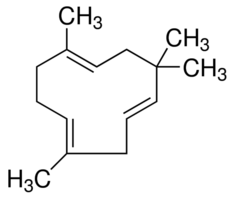
https://www.sigmaaldrich.com/catalog/product/aldrich/53675?lang=en®ion=US
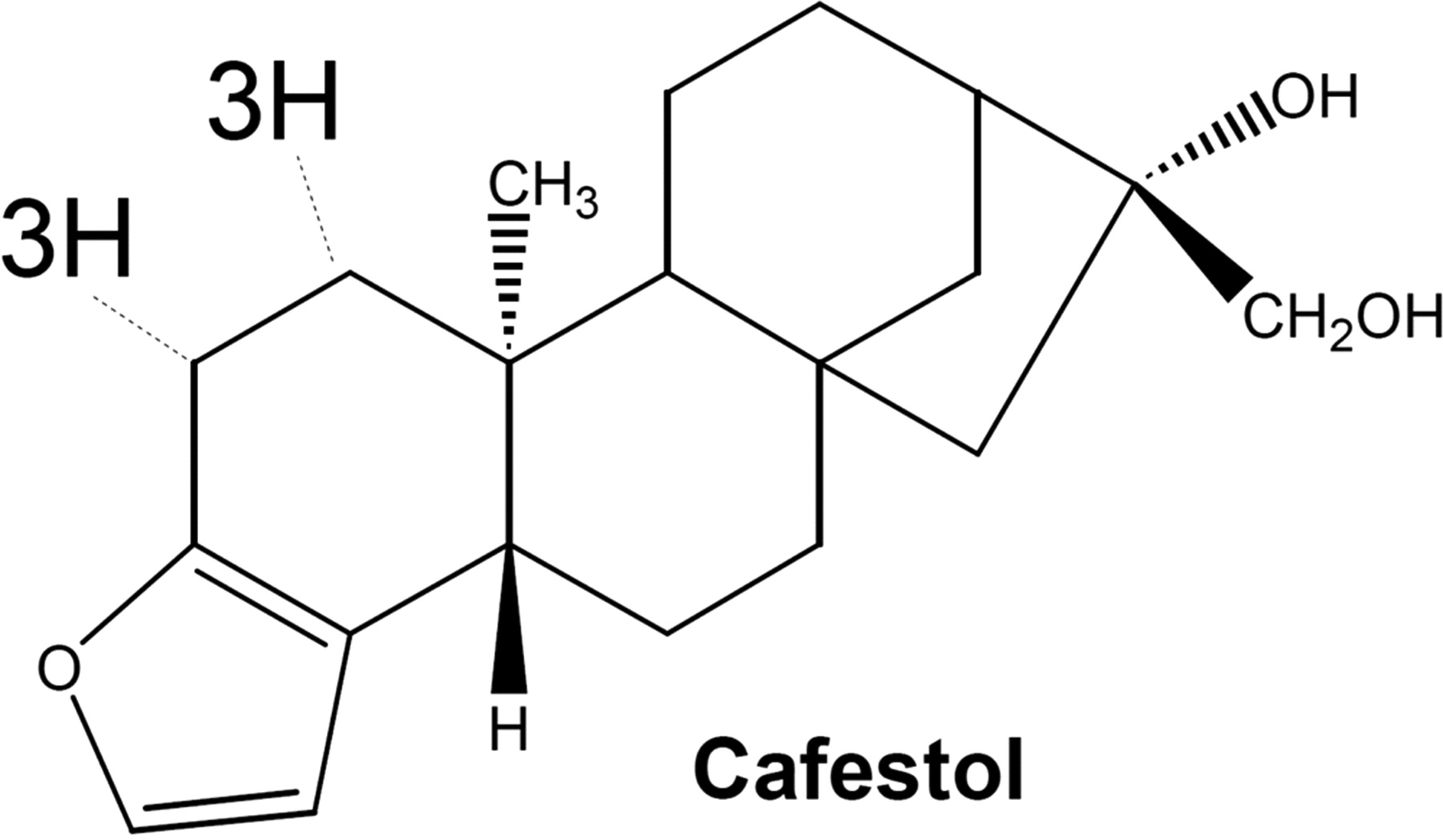
4. Diterpenoids
- 4 isoprene units present (20 Carbon).
- e.g. Ginkgolides, Cafestol (Coffee)
5. Sesterterpenoids
- 5 isoprene units present (25 Carbon).
- e.g. Geranynfarnesol, Ophiobolin
6. Triterpenoids
- 6 isoprene units present (30 Carbon).
- Found in cholesterol, vitamin.
- e.g. Squalene (Shark liver Ricebran)
7. Tetraterpenoids
- 8 isoprene units present (40 Carbon)
- e.g. Carotenoids, Lycopene
- Lycopene found in all red coloured fruits except strawberry.
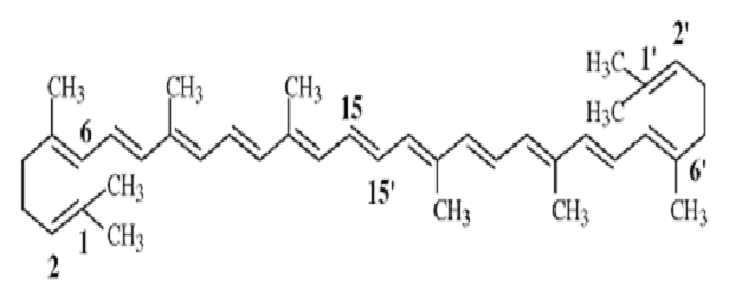
Lycopene
https://www.researchgate.net/figure/The-structure-of-lycopene_fig1_234164549
8. Polyterpenoids
- With a larger number of isoprene units.
- e.g. Natural Rubber.
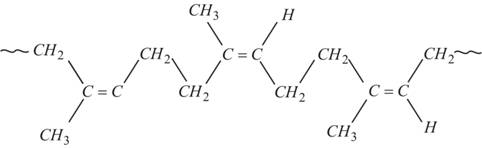
http://www.chemistrylearning.com/rubber/
Biosynthesis of Terpenoids
Biosynthesis of Terpenoids may be studied in 2 parts:
- Synthesis of activated 5-C units
- Condensation of activated 5-C units IPP and DPP to form terpenes
A. Synthesis of activated 5-C units
Isoprene unit is almost entirely synthesized from acetyl- CoA through mevalonic acid pathway. The steps of this pathway –
- Firstly, 3 acetyl-CoA molecules are jointed together in stepwise manner to form a G-C intermediate mevalonic acid.
- Secondly, Mevalonic acid is then pyrophosphated utilizing 2ATP molecules to form mevalonic acid pyrophosphate (MVA- PP)
- Thirdly, Decarboxylation and dehydration bof MVA- PP result in the formation of activated 5-C unit called as Isopentenyl pyrophosphate (IPP). The later can be isomerized to another activated 5-C unit called as Dimethylally pyrophosphate (DPP). Both these activated 5-C units are building blocks of terpenes in plant.
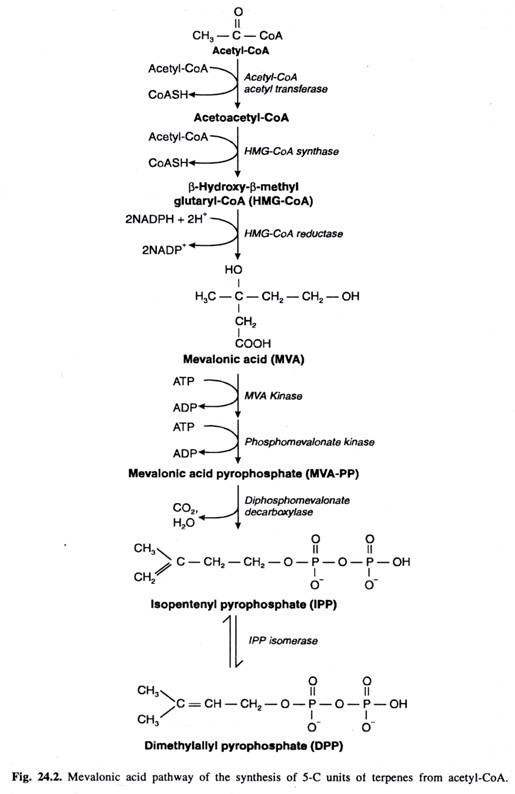
http://www.biologydiscussion.com/plant-physiology-2/stress-physiology/terpenes-classification-and-biosynthesis-with-diagram/23682
B. Condensation of activated 5-C units IPP and DPP to form terpenes
Terpenes are ultimately formed by condensation of activated 5-C units IPP and DPP.
- IPP and DPP units to form 10-C geranyl pyrophosphate (GPP) which is precursor of monoterpenes.
- GPP units with another molecule of IPP to give rise to 15-C farnosyl pyrophosphate (FPP) which is precursor of sequiterpenes.
- FPP units with IPP to form 20-C compound geranyl geranyl pyrophosphate (GGPP) which is precursor of diterpenes.
- FPP dimerises to form 30-C compound which after elimination of 2 pyrophosphate groups (2PP) gives rise to sequalene. The later is precursor of triterpens and steroids.
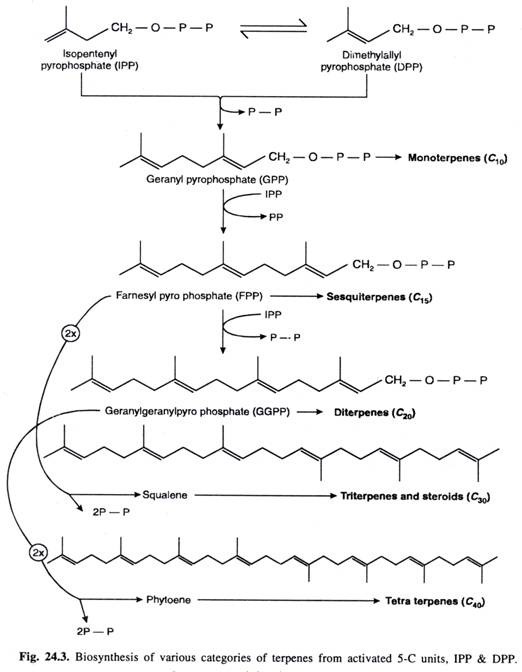
http://www.biologydiscussion.com/plant-physiology-2/stress-physiology/terpenes-classification-and-biosynthesis-with-diagram/23682 - GGPP can dimerise to form 40-C compound which after elimination of 2 pyrophosphate groups (2PP) gives rise to phytoene. The later is precursor of tetraterpenes.
- Polyterpenes are polymers containing large number of Isopentenyl units.
References & Other Links
- www.slideshare.net
- www.terpenoids.net
- www.dictionary.com
- Class lecture of Rifat Samad Mam, Assistant Professor, Department of Botany, University of Dhaka
Written by
Tubaia Zannat Juthi, B.S. (Hons), Department of Botany, University of Dhaka
Best safe and secure cloud storage with password protection
Get Envato Elements, Prime Video, Hotstar and Netflix For Free
 Plantlet The Blogging Platform of Department of Botany, University of Dhaka
Plantlet The Blogging Platform of Department of Botany, University of Dhaka





