Essential Oil
- Essential oil is a concentrated hydrophobic liquid containing volatile aroma compounds from plants.
- They are usually lipophilic (literally: oil-loving) compounds that usually are not miscible with water. Also, they can be diluted in solvents like pure ethanol and polyethylene glycol.
- They are also known as volatile oils, ethereal oils or aetherole.
- They carry a distinctive scent or essence of the plant.
- They are used in perfumes, cosmetics, soaps, lotions and other products. For flavoring food and drinks and for adding scents to incense and household cleaning products.
- It is used medicinally for skin treatments to remedies for cancer.
- Essential oils are derived from various sections of plants barriers – all spices, juniper etc.
| Seeds | Almond, cumin |
| Bark | Cassia |
| Leaves | Basil, cinnamon, lemon grass |
| Flowers | Cannabis, clove, chamomile, lavender |
| Wood | Sandalwood |
| Rhizomes | Valerian |
| Pell | Orange |
- Families particularly rich in essential oils includes Compositae (Sunflower), Labiataceae/Lamiaceae (mint), Oleaceae, Myrtaceae (eucalyptus all sp.) etc.
- Some best essential oils are lemon oil, cinnamon oil, clary sage, lemongrass oil, lavender oil, tea tree oil, eucalyptus.
Classification of Essential Oils
Best safe and secure cloud storage with password protection
Get Envato Elements, Prime Video, Hotstar and Netflix For Free
Best Money Earning Website 100$ Day
#1 Top ranking article submission website
Essential oils can be divided into 2 groups-
- Monoterpenes
- Sequiterpenes
1. Monoterpenes
- They contain 10 Carbon atoms.
- They are built up of 2 isoprene units.
- They are open ring.
- Simple monoterpenes are widely spread.
- They tend to occur as component of majority of essential oils.
Monoterpenes can be further divided into 2 groups. They are:
1. Monocyclic Monoterpenes: e.g. Limnonene, menthol, thymol etc. Menthol is a chief monoterpene compound and source of pipermint oil.
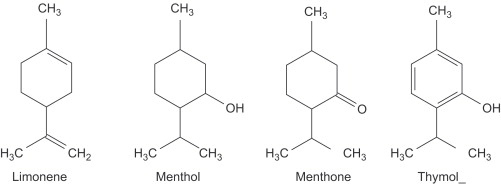
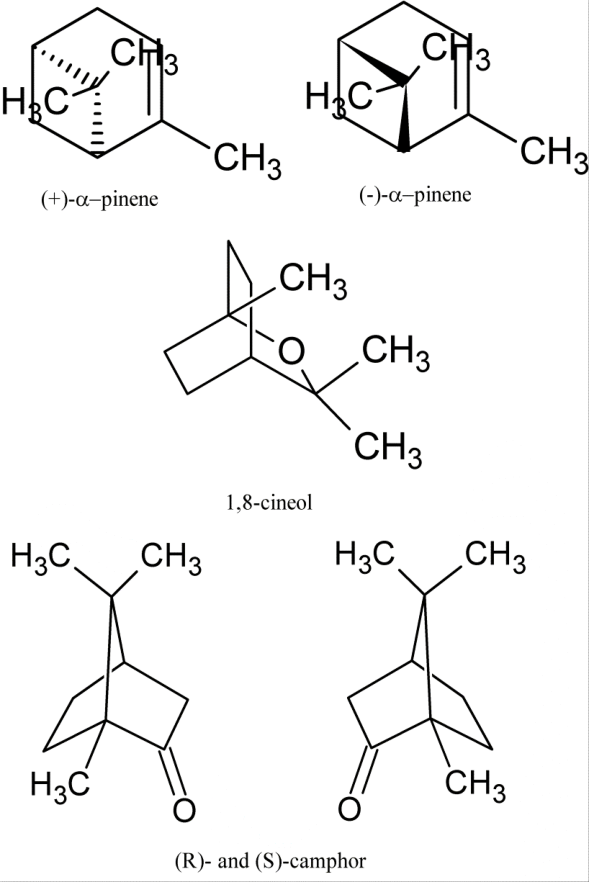
2. Bicyclic Monoterpenes: e.g. α-pinene, β-pinene, camphor etc.
2. Sequiterpenes
- They contain 15 Carbon atoms.
- They consist of 3 isoprene units.
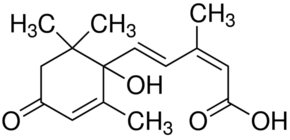
Abscisic Acid
https://www.sigmaaldrich.com/catalog/substance/2cis4transabscisicacid264321437545211?lang=en®ion=US
According to the basic Carbon skeleton, they can be further divided into 3 groups. They are-
- Acyclic sequiterpenes
- Monocyclic sequiterpenes (Abscisic Acid)
- Bicyclic sequiterpenes
References & Other Links
- www.slideshare.net
- www.terpenoids.net
- www.dictionary.com
- Class lecture of Rifat Samad Mam, Assistant Professor, Department of Botany, University of Dhaka
Written by
Tubaia Zannat Juthi, B.S. (Hons), Department of Botany, University of Dhaka
Best safe and secure cloud storage with password protection
Get Envato Elements, Prime Video, Hotstar and Netflix For Free
 Plantlet The Blogging Platform of Department of Botany, University of Dhaka
Plantlet The Blogging Platform of Department of Botany, University of Dhaka





