Proteins are an extremely important class of macromolecule in living organisms. More than 50% of the dry mass of most cells is protein. Proteins have many important functions. Amino Acids are the building units of proteins. Proteins are polymers of amino acids linked together by what is called “Peptide bond”. There are about 700 amino acids occur in nature. Only 20 of them occur in proteins. These 20 amino acids are the building blocks of proteins. All are alpha-amino acids. (2- amino acids, alpha- amino acids, or α-amino acids) They differ in respect to the group (R) attached to the alpha carbon. The amino acids obtained by hydrolysis of proteins differ in respects R (the side chain). The properties of the amino acid vary as the structure of R varies.For example:
- all enzymes are proteins
- proteins are essential components of cell membranes, their functions in membranes, such as receptor proteins and signalling proteins.
- some hormones are proteins – for example, insulin and glucagon.
- the oxygen-carrying pigments haemoglobin and myoglobin are proteins
- antibodies, which attack and destroy invading microorganisms, are proteins
- collagen, another protein, adds strength to many animal tissues, such as bone and the walls of arteries
- hair, nails and the surface layers of skin contain the protein keratin
- actin and myosin are the proteins responsible for muscle contraction
- proteins may be storage products – for example, casein in milk and ovalbumin in egg white.
Despite their tremendous range of functions, all proteins are made from the same basic monomers. These are amino acids.
Amino Acid
Each amino acid has 4 different groups, attached to α- carbon (which is C-atom next to COOH group).
These 4 groups are :
- amino group (—NH2),
- Carboxyl group ( -COOH),
- Hydrogen atom
- Side Chain (R)
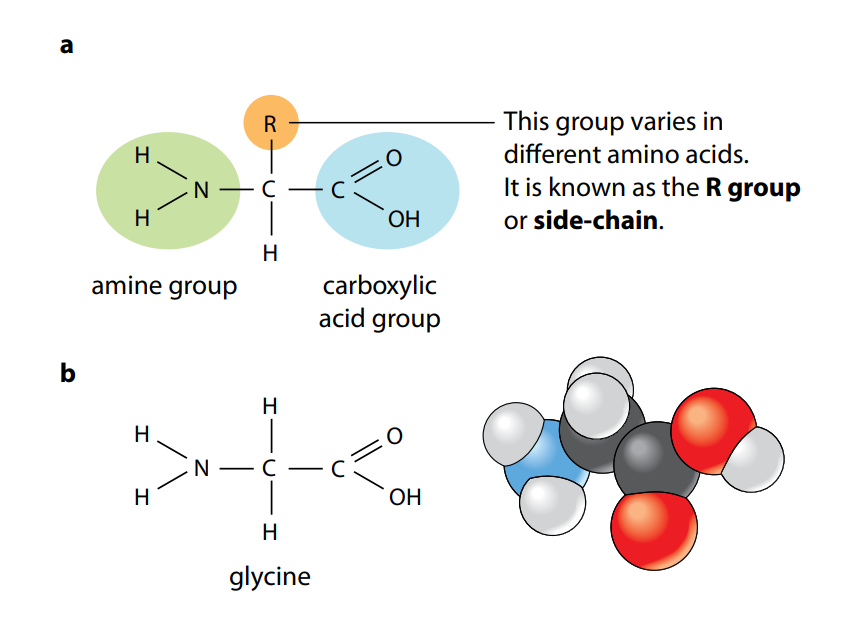
Figure A shows the general structure of all amino acids and of glycine, the simplest amino acid. They all have a central carbon atom which is bonded to an amine group, –NH2, and a carboxylic acid group, –COOH. It is these two groups which give amino acids their name. The third component that is always bonded to the carbon atom is a hydrogen atom.
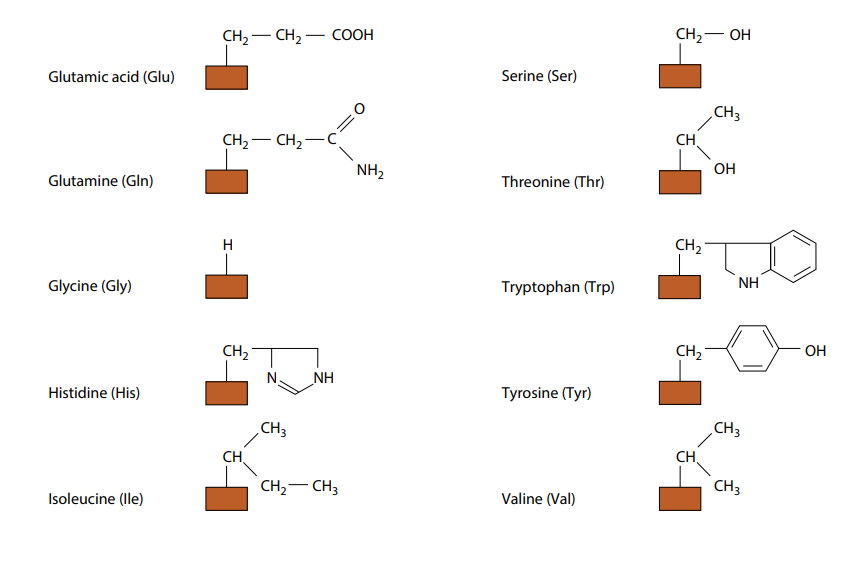
The only way in which amino acids differ from each other is in the remaining, fourth, group of atoms bonded to the central carbon. This is called the R group. There are 20 different amino acids which occur in the proteins of living organisms, all with a different R group. Their molecular formulae in Fig B.
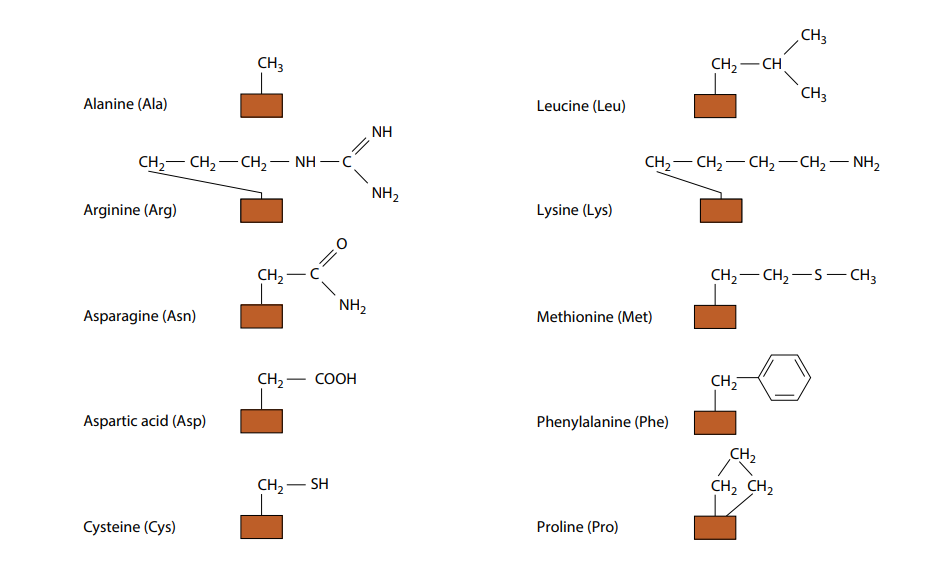
(need not to remember all the different R groups.) Fig B also shows the three-letter abbreviations commonly used by scientists for the names of the amino acids. Many other amino acids have been synthesized in laboratories.
According to polarity of side chain (R) there are 4 types of amino acids,
- Non polar amino acids
- Uncharged Polar amino acids
- Basic amino acids
- Acidic Amino acids
1.Non polar amino acids
- R is hydrophobic group (non-polar) which can’t form hydrogen bond with other molecules.
- 9 amino acids are non polar ( glycine, alanine, valine, leucine, isoleucine, phenyl alanine, tryptophan, proline and methionine).
- (Monobasic, monocarboxylic amino acids neutral or uncharged).
2. Uncharged sidechain Polar amino acids
- R contains polar hydrophilic group so can forms hydrogen bond with H2O or other atoms.
- In those amino acids, R may contain:
1- OH group (hydroxyl): as in serine, threonine and tyrosine
2- SH group (sulfhydryl) : as in cysteine (It can form disulfide bond )
3- NH2CO(amide) group: as in glutamine and aspargine - (Monobasic, monocarboxylic amino acids neutral or uncharged)
Glycine: R= H(simplest amino acid)
Alanine: R= CH3
3. Basic amino acids
- Whole amino acid contains two or more NH2 groups or nitrogen atoms that act as base and thus can bind with proton.
- R contains one or more NH2 groups or nitrogen atoms
- At physiological pH, basic amino acids will be positively charged. (Polar)
Examples
- Lysine
- Arginine: contains guanido group NH2-C=NH2
- Histidine: is an example on basic heterocyclic amino acids, Imidazole ring , blood buffer or biological buffer.
4. Acidic Amino acids
- Whole amino acid contains two or more COOH groupsthat act as acid and can donate proton.
- R contains one or more COOH groups
- At physiological pH acidic Amino acids will be negatively charged. (Polar)
- Example: Aspartic acid (aspartate) and Glutamic acid (glutamate).
- Aspargine and Glutamine: They are amide forms of aspartate and glutamate in which side chain COOH groups are amidated. So, Aspargine and Glutamine are classified as neutral amino acids.
According to nutritional value there are 3 types of amino acids,
1- Essential amino acids
These amino acids can’t be formed in the body and so, it is essential to be taken in diet. Their deficiency affects growth, health and protein synthesis.
2- Semiessential amino acids
These are formed in the body but not in sufficient amount for body requirements especially in children.
Summary of essential and semiessential amino acids
Villa HM = Ten Thousands Pound
V= valine i= isoleucine l= lysine l= leucine
A = arginine* H= histidine* M= methionine
T= tryptophan Th= threonine P= phenyl alanine
(*= arginine and histidine are semiessential)

3- Non essential amino acids: These are the rest of amino acids that are formed in the body in amount enough for adults and children. They are the remaining 10 amino acids.
Tyrosine is a nonessential amino acid and can be formed by the hydroxylation of phenylalanine in the liver when the intake of tyrosine in the diet is low. In a diet low in tyrosine, as much as half the ingested phenylalaninemay be converted to tyrosine in the body.
What is a standard amino acid?
Standard Amino Acid. A standard amino acid is an amino acid organisms use in the synthesis of peptides. There are twenty standard amino acids, all of which are alpha amino acids. Each standard amino acid has a three-letter symbol and a one-letter symbol.
The Peptide Bond
Figure (C) shows how two amino acids can join together. One loses a hydroxyl (–OH) group from its carboxylic acid group, while the other loses a hydrogen atom from its amine group. This leaves a carbon atom of the first amino acid free to bond with the nitrogen atom of the second. The link is called a peptide bond. The oxygen and two hydrogen atoms removed from the amino acids form a water molecule. We have seen this type of reaction, a condensation reaction, in the formation of glycosidic bonds and in the synthesis of triglycerides.
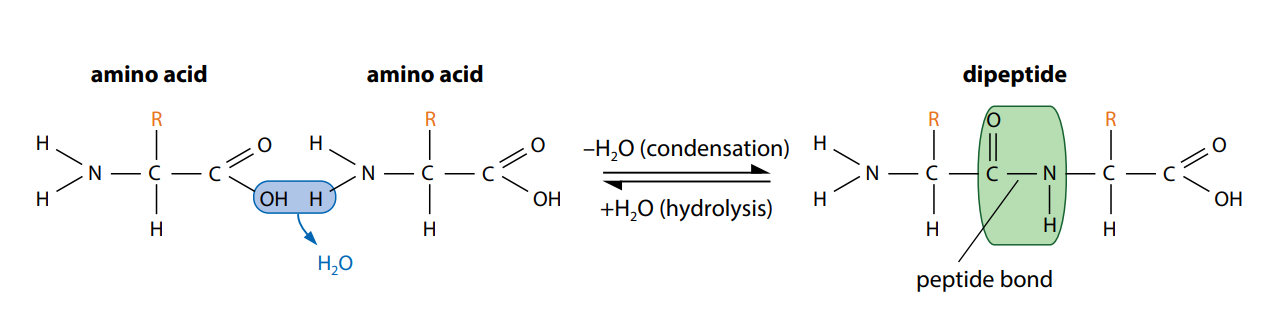
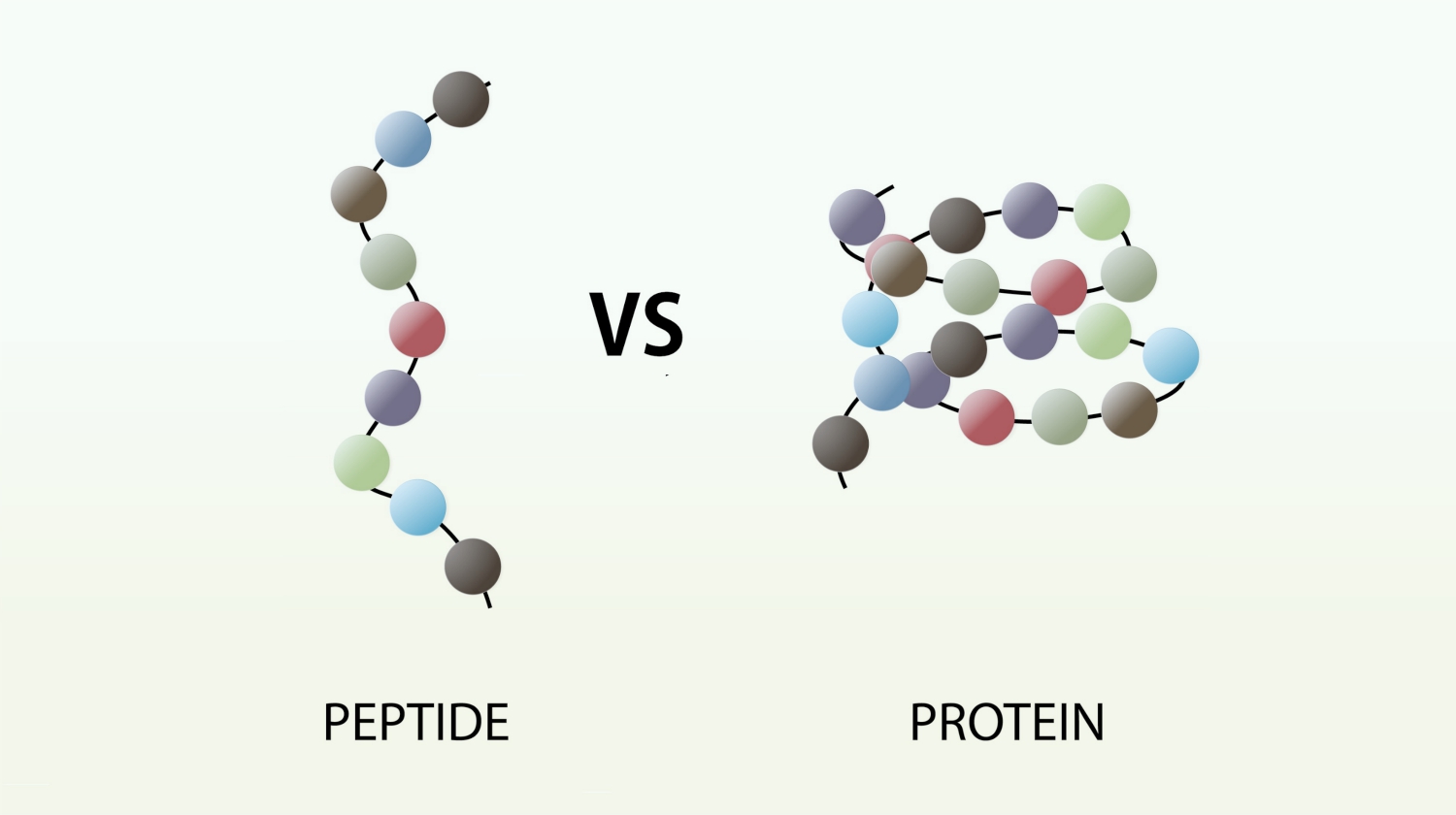
The new molecule which has been formed, made up of two linked amino acids, is called a dipeptide. Any number of extra amino acids could be added to the chain in a series of condensation reactions. A molecule made up of many amino acids linked together by peptide bonds is called a polypeptide. A polypeptide is another example of a polymer and a macromolecule, like a polysaccharide. A complete protein molecule may contain just one polypeptide chain, or it may have two or more chains which interact with each other.
In living cells, ribosomes are the sites where amino acids are joined together to form polypeptides. The reaction is controlled by enzymes.
Polypeptides can be broken down to amino acids by breaking the peptide bonds. This is a hydrolysis reaction, involving the addition of water (Figure C), and happens naturally in the stomach and small intestine during digestion. Here, protein molecules in food are hydrolyzed into amino acids before being absorbed into the blood.
Function of peptide
- Act as hormones
Insulin: Control blood glucose level
Gonadotropin-releasing hormone (GnRH). Critical for successful reproductive function.
Oxytocin is a peptide hormone and neuropeptide. It plays a role in social bonding, sexual reproduction, childbirth, and the period after childbirth.
- Antioxidant
Tripeptide:glutathione (γ-L-Glutamyl-L-cysteinylglycine).
Glutathione is capable of preventing damage to important cellular components caused by reactive oxygen species such as free radicals,peroxides, lipid peroxides, and heavy metals.
- Pain decrease
Opioid peptide.
- Neurotransmeter
Many peptides known to be hormones also act as neurotransmitters, and often these are co-released with small-molecule neurotransmitters. Acetylcholine (ACh) (substance P) and the opioid peptides, are involved in the perception of pain. Still other peptides, such as melanocyte-stimulating hormone, adrenocorticotropin, and β-endorphin, regulate complex responses to stress.
- Antimicrobial antibiotic
Valinomycin, Gramicidin A.
- Controlling blood pressure:
Angiotensin is a peptide hormone that causes vasoconstriction and an increase in blood pressure. It is part of the renin–angiotensin system, which regulates blood pressure. Ex: Angiotensin I,
Angiotensin II.
- Cell proliferation and angiogenesis regulator
Angiotensin II
- Artificial non-saccharide sweetener
Aspartame is an artificial non-saccharide sweetener 200 times sweeter than sucrose.
- Toxic: Mushroom peptides.
- Disease causing: Amyloid.
Types of peptides
1. Dipeptides:
Compound formed when two amino acids linked by 1 peptide bond.
Example: Aspartame (Asparagine-phenylalanine)
2. Tripeptides
Compound formed when three amino acids linked by 2 peptide bond.
Example: Glutathione ( Glutamyl-cystinyl-glycine)
3. Oligopeptides Compound formed when more than 2 and less than 20 amino acids are linked by many
peptide bonds.
Examples: Tetrapeptide; Tulfsin ( thrionine-lysine-proline-Arginine), Amanitin ( Decapeptide)
4. Polypeptides
Compound formed when more than 20 amino acids are linked by peptide bond.
Examples: Insulin, Growth hormone.
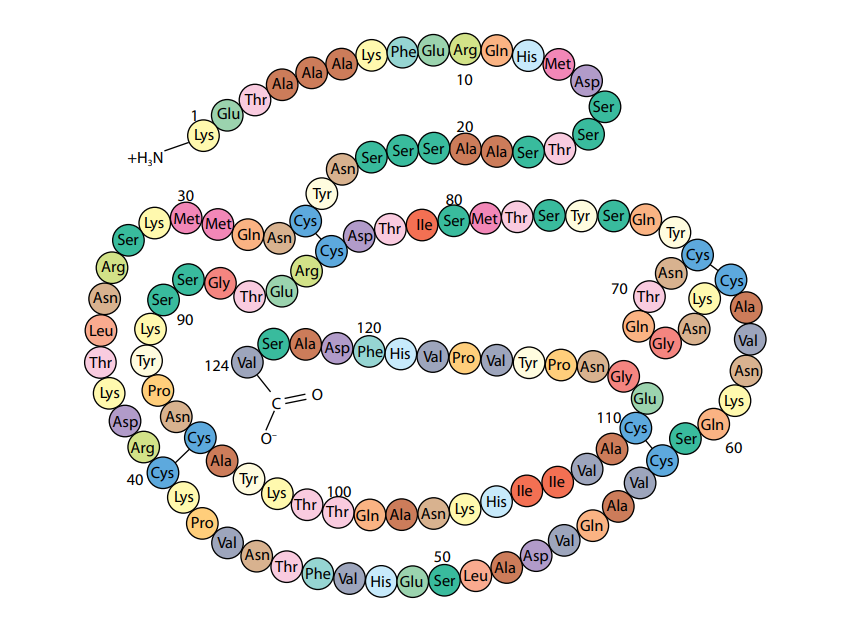
Primary Structure
A polypeptide or protein molecule may contain several hundred amino acids linked into a long chain. The particular amino acids contained in the chain, and the sequence in which they are joined, is called the primary structure of the protein. Figure (D) shows the primary structure of the protein ribonuclease, an enzyme. There are an enormous number of different possible primary structures. Even a change in one amino acid in a chain made up of thousands may completely alter the properties of the polypeptide or protein.
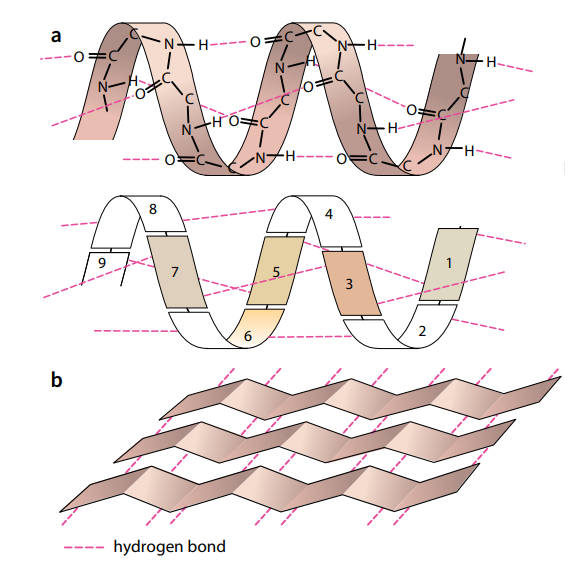
Secondary Structure
The amino acids in a polypeptide chain have an effect on each other even if they are not directly next to each other. A polypeptide chain, or part of it, often coils into a corkscrew shape called an α-helix (Figure E a). This secondary structure is due to hydrogen bonding between the oxygen of the –CO– group of one amino acid and the hydrogen of the –NH– group of the amino acid four
places ahead of it. Each amino acid has an –NH– and a –CO– group, and Figure 2.19a shows that all these groups are involved in hydrogen bonding in the α-helix, holding the structure firmly in shape. Hydrogen bonding is a result of the polar characteristics of the –CO– and –NH– groups.
Tertiary Structure
In many proteins, the secondary structure itself is coiled or folded. Figure (F) shows the complex way in which a molecule of the protein lysozyme folds. Here the α-helices are represented as coils, while in Figure (G) , which shows the secondary and tertiary structure of myoglobin, the α-helices are shown as cylinders.
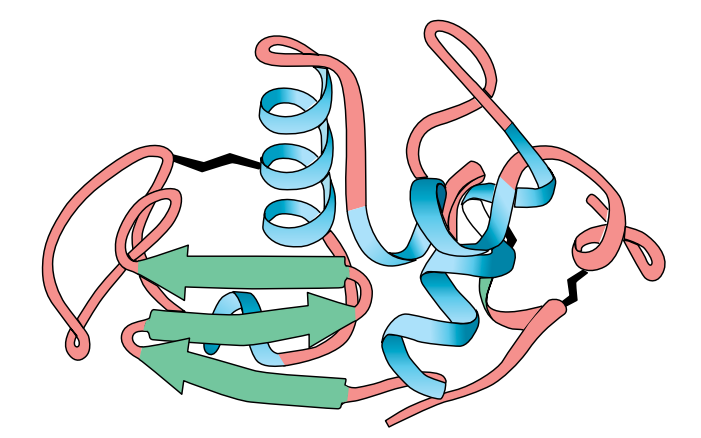
At first sight, the myoglobin and lysozyme molecules look like disorganised tangles, but this is not so. The shape of the molecules is very precise, and the molecules are held in these exact shapes by bonds between amino acids in different parts of the chain. The way in which a protein coils up to form a precise three-dimensional shape is known as its tertiary structure.
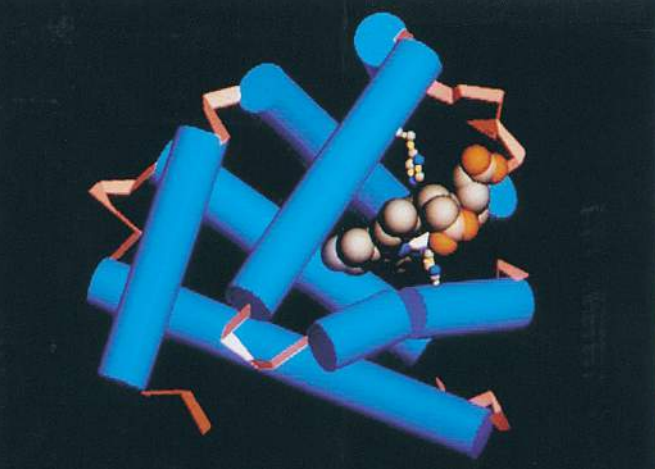
Quaternary Structure
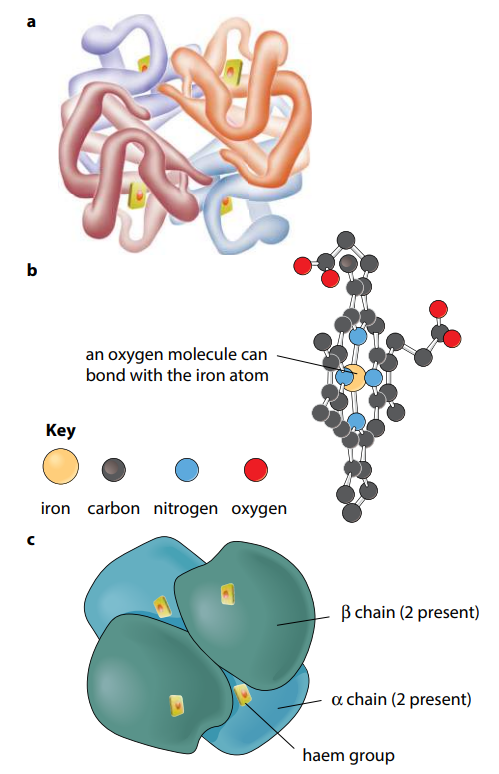
Many protein molecules are made up of two or more polypeptide chains. Hemoglobin is an example of this, having four polypeptide chains in each molecule (Figure H). Th e association of different polypeptide chains is called the quaternary structure of the protein. The chains are held together by the same four types of bond as in the tertiary structure. More details of hemoglobin are given in the next section.
So, in short, Primary structure is the sequence of amino acids in a polypeptide or protein.
Secondary structure is the structure of a protein molecule resulting from the regular coiling or folding of the chain of amino acids, e.g. an α-helix or β-pleated sheet.
Tertiary structure is the compact structure of a protein molecule resulting from the three-dimensional coiling of the already-folded chain of amino acids.
Quaternary structure is the three-dimensional arrangement of two or more polypeptides, or of a polypeptide and a non-protein component such as haem, in a protein molecule.
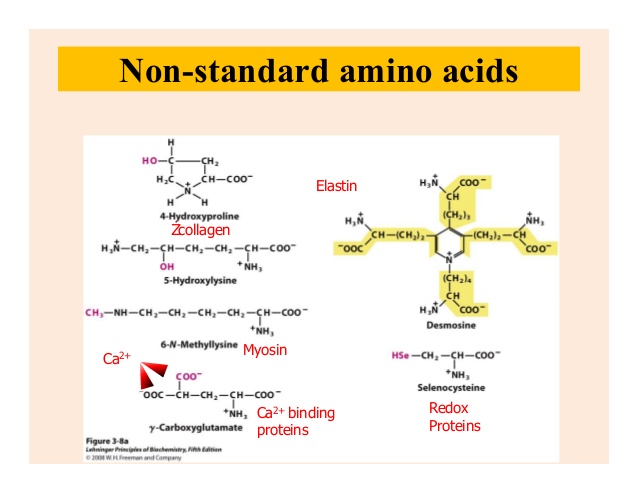
What Is Non-Standard Amino Acid?
Nonstandard amino acids refer to those amino acidsthat have been chemically modified after they have been incorporated into a protein (called a “posttranslational modification”) and those amino acidsthat occur in living organisms but are notfound in proteins.
UV absorption quality
- Eukaryotic and prokaryotic cells contain a number of compounds that are fluorescent with UV light excitation.
- Proteins and peptides, with aromatic amino acids are fluorescent when excited with UV light.
- They all contain aromatic ring structures that absorb UV light for excitation.
- To different degrees, all aromatic amino acids absorb ultraviolet light.
- Tyrosine and tryptophan absorb more than do phenylalanine.
- Tryptophan is responsible for most of the absorbance of ultraviolet light (ca. 280 nm) by proteins.
- The UV absorption of protein can be used both to quickly image and acquire spectra of microscopic samples nondestructively.
- Quantification of protein peptide and quality check of DNA.
Presence Identification and quantification of amino acids
- A ninhydrin test is a general test performed by all amino acids.
- The test is performed as a result of the reaction between the amino group of free amino acid and ninhydrin.
- Amide group also performs in this test.
- Ninhydrin is a strong oxidizing agent. Its presence causes the amino acid to go through oxidative deamination liberating ammonia and reduced form of ninhydrin.
- The formed NH3 reacts with the molecule of ninhydrin resulting in the formation of a blue substance.
- However, some amino acids like proline and hydroxyproline do not lead to the production of blue or purple substances. They usually yield to a brown colored product.
So in the end, Proteins are long chains of amino acids which fold into precise shapes. Biuret reagent can be used to test for proteins. The linkages that join amino acids are called peptide bonds. The sequence of amino acids in a protein, known as its primary structure, determines the way that it folds and hence determines its threedimensional shape and function.
Many proteins contain areas where the amino acid chain is twisted into an α-helix; this is an example of secondary structure. The structure forms as a result of hydrogen bonding between the amino acids. Another secondary structure formed by hydrogen bonding is the β-pleated sheet. Further folding of proteins produces the tertiary structure. Often, a protein is made from more than one polypeptide chain. The association between the different chains is the quaternary structure of the protein. Tertiary and quaternary structures are very precise and are held in place by hydrogen bonds, disulfide bonds (which are covalent), ionic bonds and hydrophobic interactions.
Proteins may be globular or fibrous. A molecule of a globular protein – for example haemoglobin – is roughly spherical. Most globular proteins are soluble and metabolically active. Haemoglobin contains a non-protein (prosthetic) group, the haem group, which contains iron. This combines with oxygen. A molecule of a fibrous protein – for example, collagen – is less folded and forms long strands. Fibrous proteins are insoluble. They often have a structural role. Collagen has high tensile strength and is the most common animal protein, being found in a wide range of tissues.
Reference
This Article is based on by the lecture of Farhana Tasnim Chowdhury, Lecturer, Department of Biochemistry, University of Dhaka
Some info and pictures have been added by author
Reference used for info: Cambridge International Biology Cource Book by Mary Jones.
 Plantlet The Blogging Platform of Department of Botany, University of Dhaka
Plantlet The Blogging Platform of Department of Botany, University of Dhaka





