Callus culture is a widely used technique in plant research and breeding. It involves inducing the formation of calluses, which are undifferentiated masses of cells that can be regenerated into whole plants. Establishing and maintaining calluses in tissue culture is a crucial step in many plant research studies. However, it can be challenging to cultivate healthy and robust calluses.
Broadly, there are two types of tissue cultures:
- Unorganized (callus and suspension culture)
- Organized (root cultures, shoot tip cultures, embryo cultures, etc.)
This article provides tips and techniques to help you effectively establish calluses in tissue culture.
What is Callus?
Callus is an irregular mass of tissue with meristematic loci. It lacks any organized structure but often shows cellular differentiation, mostly tracheidal elements. A well-established callus comprises different types of cells. The cellular heterogeneity of the callus is derived from the multicellular explants used to initiate callus cultures and/or induced by the culture conditions. The calli from the same explant may show considerable variation with regard to color, texture, compactness, amount of water content, and chemosynthetic and morphogenic potential.
How Callus is Developed?
In practice, a small piece of a plant organ(explant) is excised, surface sterilized, and planted on a culture medium containing an auxin and/or a cytokinin. The requirement for exogenous growth regulators for callusing varies with the explants, depending on the endogenous levels of growth regulators. 2,4-D is the most commonly used auxin to induce callus formation. On a favorable medium, the explant shows cell divisions, usually starting at the cut ends and gradually extending over the entire surface of the explant. Leading to the formation of an irregular mass of tissue, called a callus. After 3-4 weeks the callus is separated from the parent explant and transferred to a fresh medium of the same composition.
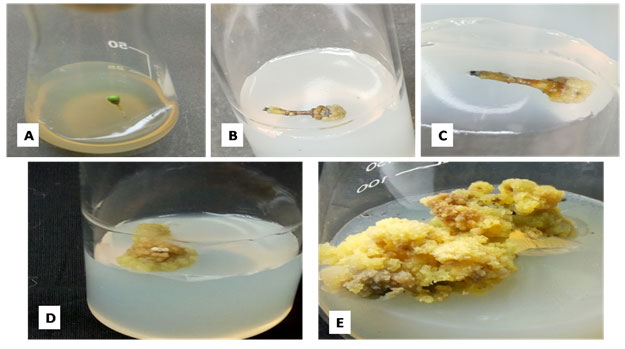
*By periodic subcultures on fresh medium, the callus can be multiplied for an unlimited period.
Establishing Callus Culture
Establishing callus culture requires the following types of equipment, materials, and conditions:
1. Explant: The plant material used for callus culture should be healthy and disease-free. The material is usually obtained by cutting small pieces of stem or leaf from the parent plant.
- Diversity (genetic) of cell types- Less differentiated cells are more responsive to callus induction on media of sample composition.
- Physiological status of the explant- callus induction from the explant will be affected by physiological status, e.g. nutrient status, hormonal content, dormancy status, etc.
- Genotype- e.g. soybean varieties vary in their requirement for cytokinins, i.e. there are cytokinin autotrophs and auxotrophs.
2. Nutrient Medium: The plant material is cultured in a nutrient-rich medium containing essential nutrients, vitamins, and growth hormones. The medium should be sterilized before use to avoid contamination.
- Mineral nutrients– essential micro and macronutrients.
- Organic constituents– ‘basal’ constituents are sucrose or glucose/fructose as carbon sources and usually inositol and thiamine-HCl. Five basic groups of callus tissue types based on growth regulator requirements are:
- Auxin and cytokinin autotrophic tissues: immature lemon fruit, genetic tumor-producing plants.
- Cytokinin autotrophic: i.e. requires auxin- cereal callus, carrot root.
- Auxin autotrophic: i.e. requires cytokinin-turnip root, carrot.
- Auxin and cytokinin auxotrophic: most dicots.
- Auxin and cytokinin auxotrophic, and require complex natural extracts: orchid seedlings.
3. Culture Environment
- Temperature: 24º to 28ºC.
- Light: Dark or diffuse light (1000 lux).
4. Sterile Working Environment: The work environment should be free of contaminants and all equipment should be sterilized to avoid inducing any microorganisms into the culture.
Process of Establishing Callus Culture
Step-1: Preparation of plant material
The plant material is cut into small pieces and sterilized using ethanol or bleach.
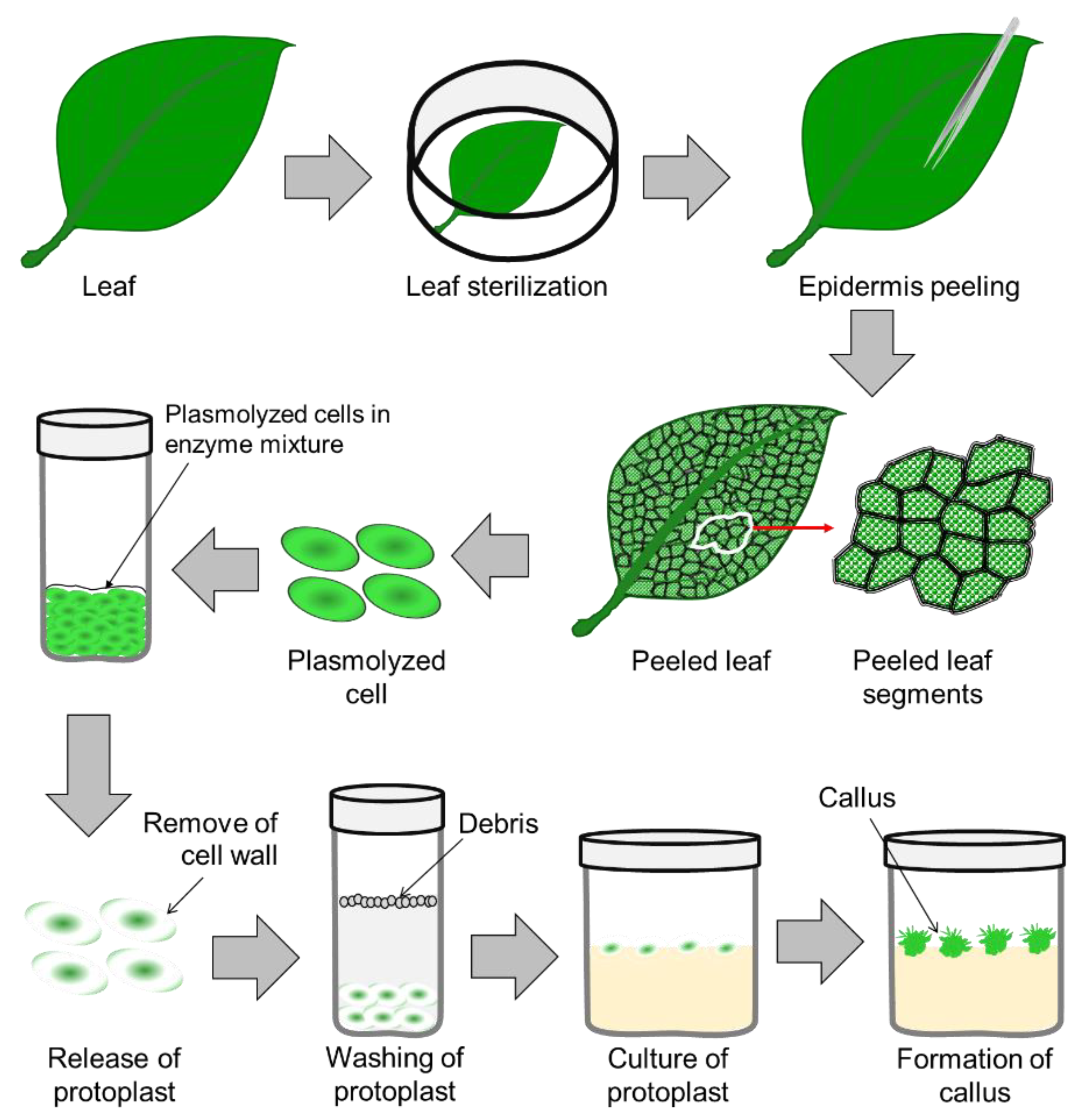
Step-2: Inoculation
The sterilized plant material is placed in the nutrient-rich medium and incubated in a dark, sterile environment.
Step-3: Observation
After a few days, the plant material will start forming a callus. The culture should be observed regularly for signs of contamination, growth, and color changes.
Q.1) How long does it take to establish calluses in callus culture?
The time it takes to establish calluses in callus culture can vary depending on the plant species, tissue type, and culture conditions. In general, callus formation can be observed within a few days to several weeks after inoculation.
However, the process of callus formation and growth is influenced by several factors such as the composition of the culture medium, the presence of growth regulators, the light intensity, temperature, and humidity. Therefore, it is difficult to provide a specific timeline for callus formation in callus culture.
Moreover, the establishment of a stable and homogeneous callus culture may require several subcultures over several months, during which time the cells are adapting to the new culture conditions and propagate through cell division. Ultimately, the time it takes to establish calluses in callus culture can vary depending on the factors mentioned above and the specific goals of the experiment.
Q.2) What is the best media composition for callus formation?
The best media composition for callus formation depends on the plant species and tissue type being cultured, as well as the desired characteristics of the callus. However, there are some general guidelines that can be followed when selecting a media composition for callus formation.
In general, media for callus formation should contain a balanced combination of macronutrients, micronutrients, vitamins, and growth regulators. The macronutrients should include a source of nitrogen, phosphorus, and potassium, as well as other essential elements like calcium, magnesium, and sulfur. The micronutrients should include trace elements like iron, zinc, and manganese.
The growth regulators added to the media play an important role in promoting callus formation. Typically, a combination of auxins and cytokinins is added to induce cell division and stimulate the formation of callus tissue. Commonly used auxins include 2,4-dichloro phenoxy acetic acid (2,4-D) and naphthaleneacetic acid (NAA), while common cytokinins include kinetin and benzyl adenine (BA). The exact concentrations of these nutrients and growth regulators can vary depending on the plant species and tissue type being cultured. Therefore, it is important to optimize the media composition through experimentation and careful observation of the culture.
Q.3) Why is it essential to maintain a sterile working environment?
A sterile working environment is essential to avoid introducing any microorganisms into the culture, which can contaminate and affect the growth of callus cells.
Q.4) What are some common contaminants in callus culture?
Some common contaminants in callus culture include:
- Bacteria: These are single-celled microorganisms that can cause discoloration of the culture medium and produce a foul odor. Common bacterial contaminants in callus culture include Pseudomonas, Agrobacterium, and Erwinia.
- Fungi: These are multicellular organisms that can cause the formation of fluffy, cotton-like growth on the surface of the culture medium. Common fungal contaminants in callus culture include Aspergillus, Penicillium, and Fusarium.
- Yeast: These are unicellular fungi that can cause the formation of cream-colored, mucoid colonies on the surface of the culture medium. Common yeast contaminants in callus culture include Candida and Saccharomyces.
-
Viruses: These are small infectious agents that can cause stunted growth, necrosis, or other abnormalities in the culture. Common viral contaminants in callus culture include tobacco mosaic virus and cucumber mosaic virus.
Q.5) Can callus be cryopreserved?
Yes, a callus can be cryopreserved. Cryopreservation is a technique used to preserve cells, tissues, or organs at extremely low temperatures (-196°C) by freezing them in liquid nitrogen. It involves adding a cryoprotective agent to the culture, which helps protect the cells from damage during freezing and thawing.
Cryopreservation of callus is useful for long-term storage and preservation of valuable genetic resources. The callus tissue is first treated with a cryoprotectant, then frozen and stored in liquid nitrogen. Cryopreserved callus cultures can be revived by thawing and re-culturing the tissue.
However, it is important to note that not all callus cultures are suitable for cryopreservation. The success of cryopreservation depends on several factors, including the type of tissue, the stage of development, and the composition of the cryoprotectant used. Therefore, it is recommended to optimize the cryopreservation protocol for each specific callus culture to ensure successful preservation.
References
- Plant Tissue Culture: An Introductory Text by SS Bhojwani
- Class notes
 Plantlet The Blogging Platform of Department of Botany, University of Dhaka
Plantlet The Blogging Platform of Department of Botany, University of Dhaka





Simply desire to say your article is as surprising. The clearness in your post is simply excellent and i could assume you are an expert on this subject. Fine with your permission let me to grab your feed to keep up to date with forthcoming post. Thanks a million and please carry on the gratifying work.
certainly like your website but you need to take a look at the spelling on quite a few of your posts. Many of them are rife with spelling problems and I find it very troublesome to inform the reality nevertheless I will definitely come back again.
Fantastic beat ! I would like to apprentice while you amend your web site, how could i subscribe for a blog site? The account helped me a acceptable deal. I had been a little bit acquainted of this your broadcast offered bright clear concept
Thank you for the good writeup It in fact was a amusement account it Look advanced to far added agreeable from you However how could we communicate
Wow, wonderful blog layout! How long have you been blogging for? you make blogging look easy. The overall look of your site is great, as well as the content!
Fantastic read! I was especially impressed by the depth provided on the topic, offering a perspective I hadn’t considered. Your insight adds significant value to the conversation. For future articles, it would be fascinating to explore more to dive deeper into this subject. Could you also clarify more about the topic? It caught my interest, and I’d love to understand more about it. Keep up the excellent work!