Soil is defined differently by soil scientists, and its definition has changed overtime. Since the year 1800, there have been more than 80 different definitions of soil available in literature.
- Generally, soil can be defined as the organic and inorganic materials on the surface of the earth that provide the medium for plant growth.
- Soil develops slowly over time.
- It is composed of many different materials like organic materials originated from living organisms and inorganic materials originated from the rocks and minerals through weathering.
Soils are mainly of 2 classes:
- Organic soil: Soils that come from living material such as plant and animal remain are organic soil.
- Mineral soil: Soils that come from rocks and other non- living materials are mineral soil.
Soil environment is important in ecology because it influences many functions of the plants. Uptake of nutrients by plants and the availability of minerals to plants are fully related to the soil environment. Therefore, growth, abundance and distribution are influenced by soil environment.
Soil environment or soil properties can be studied under two different aspects:
A) Physical aspects and
B) Chemical aspect
Best safe and secure cloud storage with password protection
Get Envato Elements, Prime Video, Hotstar and Netflix For Free
Best Money Earning Website 100$ Day
#1 Top ranking article submission website
A) Physical aspects
Soil possesses many characteristic physical properties each of which can be studied under the following headings:
(a) Thermal properties
(b) Soil temperature
(c) Soil structure and composition
(d) Soil aeration
(e) Movement of minerals through soil profiles/ Soil horizon
(a) Thermal properties
Soil receives both long wave and short wave radiation. These radiations are variously absorbed, re-radiated, reflected and also used in the evaporation of water (lost or gained by convection). Ecologists have a considerable interests in the radiation environment. These properties of soil are usually
measured as follows:
(i) Specific heat or heat capacity
(ii) Thermal heat conductivity
(i) Specific heat or heat capacity: Specific heat or heat capacity is the amount of energy needed for a given amount of soil to raise the temperature by 1oC . It is usually measured in Joules/g/oC or [J g-1 oC-1] (in SI unit formerly as Cal/g/oC).
- Specific heat of air is about 1 J g-11 oC-1 and that of water is about 4.2 Jg-1oC-1.
- The volumetric specific heat of air is about 1.2 KJ m-3 oC-1 and that of water is about 4.2×103 KJ m-3 oC -1).
Specific heat is mainly determined by its water content.
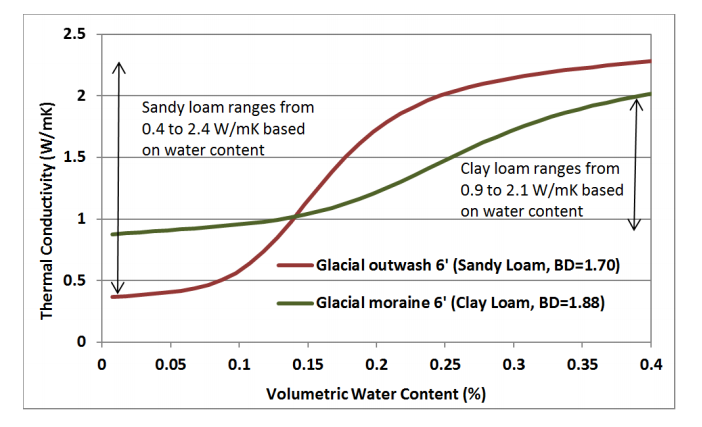
(ii) Thermal heat conductivity: Thermal heat conductivity is a measure of the ability of a substance to transfer heat and is usually expressed in Jules/s/m/oC = J.s-1m-1-oC-1 or Wm-1oC-1.
The thermal conductivity in soil air is the lowest 0.025 wm-1oC-1. The conductivity of soil minerals is about 100 times greater than this and that of water is about 20 times. So, water has to get a strong role in the conduction of heat by soil.
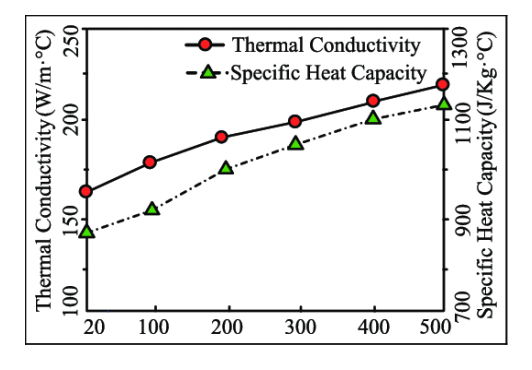
capacity of soil. Source: Unknown.
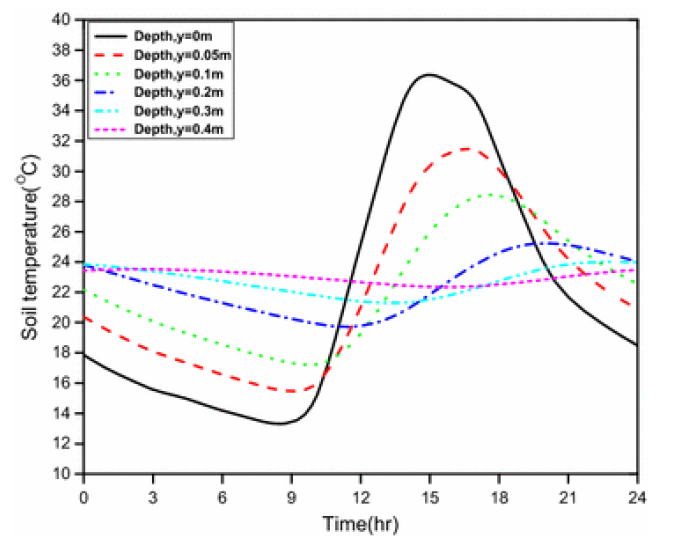
(b) Soil temperature
- During day, the maximum temperature is found at the surface and gradually decreases with depth, whereas, during night, a reverse situation may occur in the upper layer.
- The temperature of soil is usually influenced by the characteristics of its surface. e.g. by its color, texture, water content, slope, altitude of the land and also by climate and vegetational cover.
- Dark soil absorbs large amount of incidental energy whereas light soils act as reflectors.
- The germination of seeds, normal growth of roots and biological activity of soil-inhabiting micro and macro organisms require proper and specific temperature.
Soil environment: Physical aspects. Source Unknow.
(c) Soil structure and composition
- The mineral particles of soil are usually classified with regard to their size.
- The relative contribution of sand, silt and clay to the mechanical composition of soil determines the textural classification of soil.
- The texture of a soil is determined by the size of soil particles.
- A good mixture of sand, silt and clay is called loams (40% sand, 40% silt and 20% clay).
- A sandy loam has more than 50% of sand.
- Soil texture influences drainage, aeration, moisture retention.
- Soil texture also has an interactive effect on soil temperature through variation in soil moisture content: wet soil is colder and takes longer time to absorb heat from surrounding than dry soil which absorbs heat faster.
- The proportion of air to water among the soil particles decrease with the decrease in particle size, so that clay, which is fine textured, have a high water content and low air content, in turn, can inhibit root respiration and water absorption.
| Size classification of soil mineral particles according to the system of International Society of Soil Science | |
| Diameter of the particles | Name of particles |
| Less than 0.002 mm | Clay |
| Between 0.002 to 0.02 mm | Silt |
| Between 0.02 to 0.2 mm | Fine sand |
| Between 0.2 to 2 mm | Coarse sand |
(d) Soil aeration
- Soil air is of different composition to the atmosphere. Its nitrogen content is apparently same but the balance between CO2 and O2 varies.
- In the atmosphere, the concentration of CO2 is 367ppm and concentration of CO2 in soil is 10 to 100 times greater than this.
- If temperature is increased and organic matter is added, CO2 conc. will also increase.
- When reducing condition occurs in the soil, various gases such as NH4, CH2=CH2, NH3, H2S may occur. These may be injurious to the plant.
(e) The movement of matter in soil
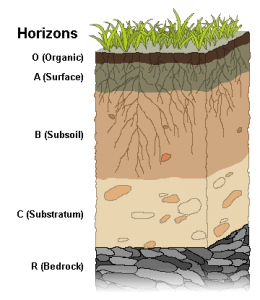
The vertical movement of water and leaching and deposition of materials occurs in soil. As a result, there is a formation of distinct horizontal layers known as horizons. These horizons are labeled alphabetically from the soil surface:
• ‘A’ horizon = Leaching of some material
• ‘B’ horizon = Zone of redeposition
• ‘C’ horizon = Unaffected by the process of soil formation
• ‘R’ horizon = Underlying rock
Besides, there are other horizons:
• O2 = Litter
• O1 = Partly decomposed litter
• A1 = Organic stained
• A2 = Bleached sand
• ‘O’ horizon i.e. organic horizon, is formed above the ‘A horizon
B) Chemical aspects of soil/Soil reactions
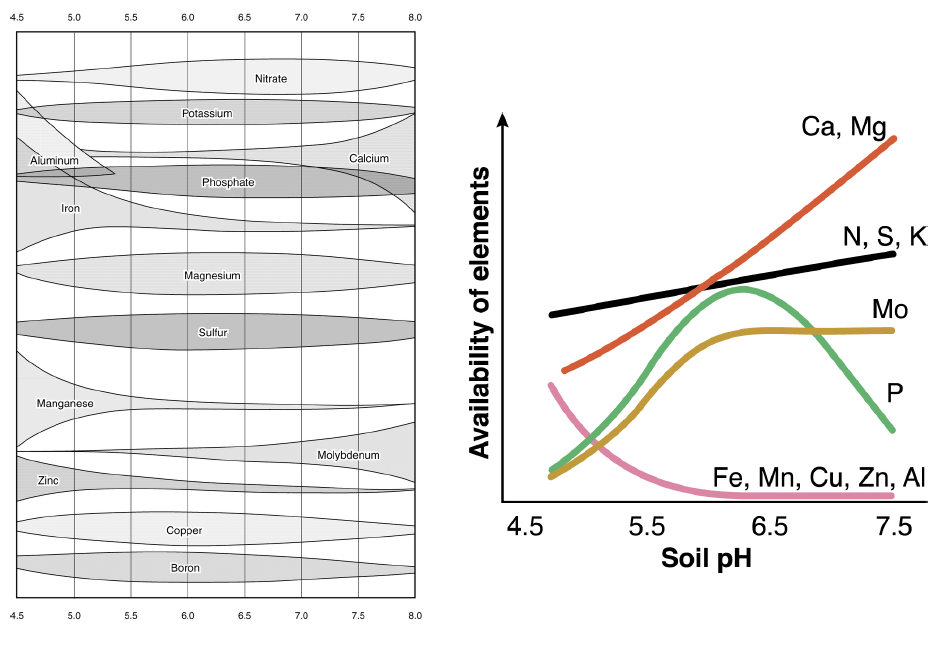
(a) Soil pH
- The acidity and alkalinity of a soil is usually influenced by pH.
- The pH of most mineral soils lies between 3.5 and 10.0, although peat soils may be less than 3.0 and alkaline or saline soils are of as high as 11.0.
- Acid substrates support relatively few species whereas calcareous ones usually exhibit considerable plant diversity.
- Waterlogged soils show a decreasing tendency of pH when they dry out, reduced compounds such as sulphide is oxidized to form sulfate, as a result there is a formation of H2SO4. Therefore, the pH of waterlogged soil decreases.
The principal effect of soil acidity on plant is concerned with the uptakes of nutrients by roots. This can be influenced in two ways:
(a) through hydrogen ions competing for ion exchange sites and
(b) indirectly by influencing the availability of certain number of principal plant nutrients and also the presence of toxic ions.
- As pH increases, the amount of exchangeable Ca, Mg, P, B and Mo in the soil increases but the availability of Fe, Mn and Zn decreases.
- However, at low pH value such as 5.0, Fe, Mn and Al levels increase resulting toxicity to plants specially when the soil is waterlogged and poorly aerated for part of the year.
- Overall nutrient availability occurs in soils at a pH of about 6.5.
- Bacterial and fungal activity in soil is also affected by pH.
- Activity is at a maximum between 5.0 and 11.0 and throughout this range decomposition of organic matter proceeds rapidly.
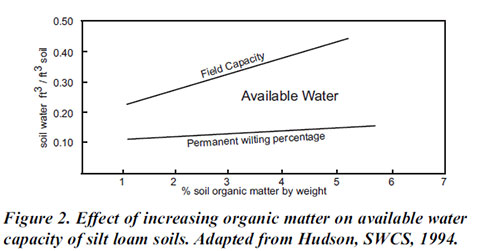
(b) Soil organic matter
Soil organic matter is mostly derived from the vegetation and is broken down by bacteria and fungi, assisted by the activities of larger animals such as earthworms.
- The microorganisms may release inorganic materials from the organic materials through a process known as mineralization.
- The presence of organic matter increases the capacity of soil to hold cations and water.
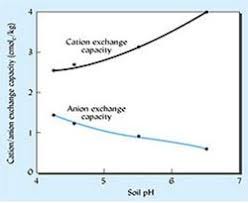
(c) Ion exchange
Most of the readily available ions in the soil are attached to soil particles, although some are in solution. Clay and organic particles are negatively charged and thus can bind cations. Soil also have an ability to hold anions.
- The ability to hold cations is usually expressed in terms of milliequivalents per unit soil. The exchange capacity is made up of total exchangeable hydrogen (TEH) and total exchangebale bases (TEB).
i.e. exchange capacity = TEH+TEB - Exchange capacity is often associated with the organic content of a soil and (as acidic soils are often organic) may decrease with increasing pH.
 Plantlet The Blogging Platform of Department of Botany, University of Dhaka
Plantlet The Blogging Platform of Department of Botany, University of Dhaka





