Units of work
Whenever a new quantity is introduced in physics, the standard metric units associated with that quantity are discussed. In the case of work (and also energy), the standard metric unit is the Joule (abbreviated J). One Joule is equivalent to one Newton of force causing a displacement of one meter. In other words,
In fact, any unit of force times any unit of displacement is like a unit of labor . Some nonstandard units for work are shown below. Notice that when analyzed, each set of units is like a unit of measurement times a volume unit .
Best safe and secure cloud storage with password protection
Get Envato Elements, Prime Video, Hotstar and Netflix For Free
Best Money Earning Website 100$ Day
#1 Top ranking article submission website
Units of Heat
SI Unit of Heat
Usually, within the System International d ‘Unites , all sorts of energy are measured in terms of joules. Notably, heat may be a sort of energy, and thus the SI unit of warmth is additionally joules (J) which are defined because the amount of energy needed to boost the temperature of a given mass by one degree. Usually, 4.184 joules of warmth energy is important to extend the temperature of a unit weight (say 1 g) of water from 0 degrees to 1 degree Centigrade .
Other Heat Units
In the cgs , heat is expressed within the unit of calories which is further said to be the warmth energy needed to extend the temperature of 1 gm of unpolluted water by one degree Celsius. Sometimes kilocalorie (kcal) is additionally mentioned as a unit of warmth where 1 kcal = 1000 cal.
Additionally, British thermal unit (BTU), which is a component of the imperial system, is additionally wont to measure or calculate heat.
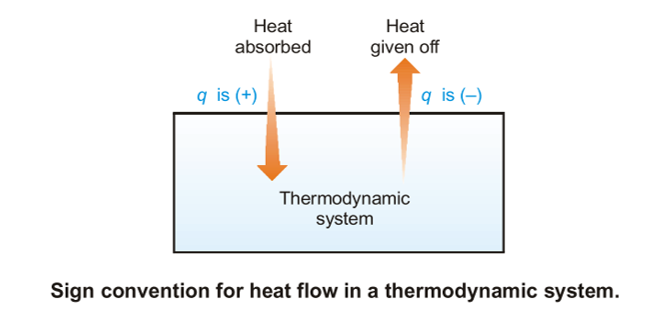
Sign Convention of Heat
The signs of ‘w’ and ‘q’ are associated with the interior energy change. When ‘w’ or ‘q’ is positive, it means energy has been supplied to the system as work or as heat. the interior energy of the system in such a case increases. On the opposite hand, if ‘w’ or ‘q’ is negative, it means energy has left the system as work or heat. the interior energy of the system decreases. The signs of ‘q’ and ‘w’ are:
- Heat absorbed by the system= q positive (+)
- Heat evolved by the system= q negative (-)
- Work done on the system= w positive (+)
- Work done by the system= w negative (-)
Isothermal Reversible Expansion Work of an Ideal gas
The commonest example of labor within the systems discussed during this book is that the work of expansion. it’s also convenient to use the work of expansion to exemplify the difference between work that’s done reversibly which which is completed irreversibly. the instance of expansion against a continuing external pressure is an example of an irreversible pathway. It doesn’t mean that the gas can’t be re-compressed. It does, however, mean that there’s a particular direction of spontaneous change in the least points along the expansion.
Imagine instead a case where the expansion has no spontaneous direction of change as there’s no net force push the gas to hunt a bigger or smaller volume. the sole way this is often possible is that if the pressure of the expanding gas is that the same because the external pressure resisting the expansion in the least points along the expansion. With no net force pushing the change in one direction or the opposite , the change is claimed to be reversible or to occur reversibly. The work of a reversible expansion of a perfect gas is fairly easy to calculate.
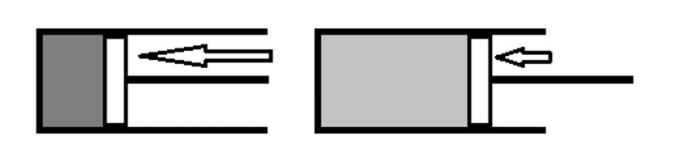
If the gas expands reversibly, the external pressure (Pext) are often replaced by one value (pp) which represents both the pressure of the gas and therefore the external pressure.
w= −∫pdV
But now that the external pressure isn’t constant, pp can’t be extracted from the integral. Fortunately, however, there’s an easy relationship that tells us how pp changes with changing VV – the equation of state! If the gas is assumed to be a perfect gas.
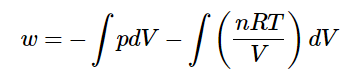
And if the temperature is held constant (so that the expansion follows an isothermal pathway) the nRT term are often extracted from the integral.
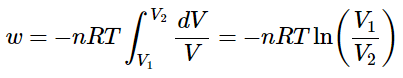
The upper equation springs for ideal gases only.
Conclusion
So, within the end we live within the century of technology and science where the introduction of science in our daily activities has transformed our lives. during this era , we are completely hooked in to physics and that we cannot deny this fact. Physics helps us in several things like maintaining our health, transporting people and goods, providing energy constructing buildings, helping us communicate, stimulating the economy and far more. Hence, we will say that physics may be a vital think about our lifestyle and thermodynamics one among the foremost important topics in physics.
Thermodynamics is as old because the universe itself, and therefore the universe is just the most important known thermodynamic system. Mainly, it’s all about energy i.e. how energy gets used and the way it changes from one form to a different . With thermodynamics, you’ll determine how efficient things are using energy for useful purposes, like generating electricity, moving an airplane, or maybe riding a bicycle. The word thermodynamics features a Greek heritage. the primary part thermos, conveys the thought that heat is somehow involved and therefore the second part dynamics, causes you to consider things that move.
Application of First Law of Thermodynamics in Daily life
- Light bulbs transform electricity into light energy (radiant energy).
- One ball hits another, transferring K.E. and making the second ball move.
- Plants convert the energy of sunlight into energy stored in organic molecules.
- You are transforming energy from your last snack into K.E. as you walk, breathe, and move your finger to scroll up and down this page.
Application of Second Law of Thermodynamics in Daily life
- Jet engines
- Spark-ignition engines
- Fossil-fueled steam power plants
- All the refrigerators
- Deep freezers
- Industrial refrigeration systems
- All types of air-conditioning and air-cooling systems
- Heat pumps and others
Application of Third Law of Thermodynamics in daily life
- The direct use of the Third Law of Thermodynamics occurs in ultra-low temperature chemistry and physics.
- The applications of this law are wont to predict the behavior of various materials to temperature changes. These relationships became core to several scientific disciplines, although the Third Law of Thermodynamics isn’t directly utilized the maximum amount because the other two.
We can conclude that thermodynamics is a crucial a part of our lifestyle . The second law of thermodynamics plays the foremost important role in making our life easier( heat transfer), which relates to transfer of warmth between two mediums. There are three modes of warmth transfer: conduction, convection and radiation. The concept of warmth transfer is employed in wide selection of devices like heat exchangers, evaporators, condensers, radiators, coolers, heaters, etc. These are a number of the foremost important objects we use in our day to day life.
Reference
This Article is based on by the lecture of Dr. Md. Ismail Hosen,
Assistant Professor, Department of Biochemistry and Molecular Biology
University of Dhaka.
Some info and pictures have been added by Author and from Wikipedia.
Reference used for info: Cambridge International AS and A Level Physics Coursebook.
 Plantlet The Blogging Platform of Department of Botany, University of Dhaka
Plantlet The Blogging Platform of Department of Botany, University of Dhaka





