The term ‘soil solution‘ refers to the liquid phase of soil which consists of dissolved substances both organic and inorganic, in ionic or in molecular form. It is the focal point of soil chemistry. Its importance in soil development, nutrient availability, and plant growth can broadly be divided into two aspects- edaphological and pedological.
- Edaphology: The study of soil related to plant and plant growth.
- Pedology: The study of soil related to soil formation.
Edaphological Importance 0f Soil Solution
1. Serves as a source of nutrients for plants-
The main function of soil solution is to supply mineral nutrients to the growing plants. Plants absorbs essential elements from soil solution for their growth. The supply of nutrients in the solution is continuously replenished by soil colloids as plants absorb them.
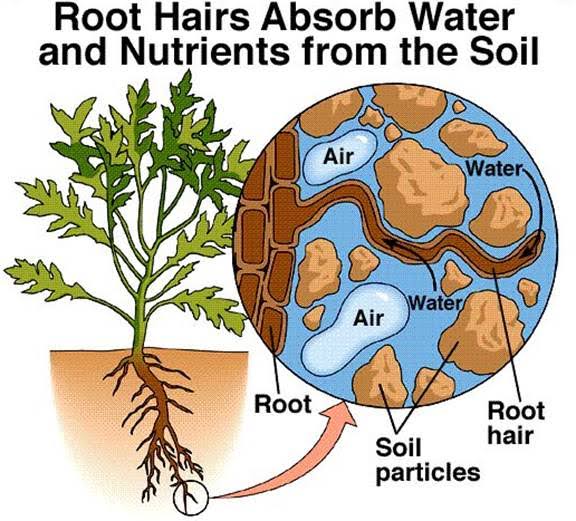
2. Serves as a source of nutrients for microorganisms-
The soil solids, particularly the fine organic and inorganic particles, release essential elements to the soil solution, from which the micro-organisms take up nutrients for their growth and reproduction. Micro-organisms also plays an important role on the release of nutrients through decomposition of organic matter. Besides, dissolved oxygen in soil solution plays a vital role for life cycle or growth of micro-organisms especially aerobic micro-organisms.
3. Is the medium where most of the chemical and biochemical reactions occur-
Many chemical and biochemical reactions are dependent on the levels of H+ and OH- ions in the soil solution, i.e. the pH of the soil solution. The pH influence the solubility, and in turn the availability of several essential elements (including Fe and Mn) to plants.
(CO2 present in soils produce carbonic acid which affects the pH and solubility of minerals.)
4. Is the medium for transport of nutrients as well as pollutants-
The nutrients present in soil solution move towards the roots of plants and within the soil by mass flow, diffusion, and root interception. Some pollutants coming from different sources contaminate the solution and moves along soil polluting it. (Arsenic, lead, heavy metals)
5. Controls the water availability and may cause salt injury to plants.
i. Water availability
The potential of water molecules to move from a lower concentrated area to a higher concentrated area by osmosis is commonly known as osmotic potential. It is also called solute potential (Ψs). The osmotic potential is zero in distilled water.
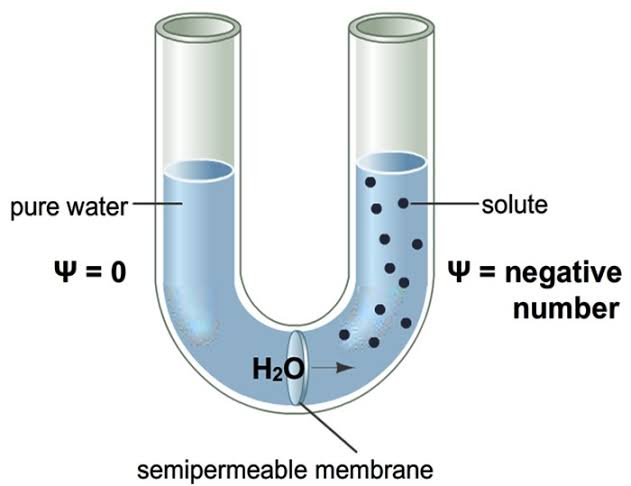
In the plant cell, the concentration of cytoplasm is higher than the distilled water. So it’s osmotic potential is always negative. Thus a difference is created between the concentration of distilled water and plant cell. Because of this difference in the value of osmotic potential, water will move from the soil into a plant’s root cells.
In reality, the water of soil is not distilled. Many substances especially salts are dissolved in the soil water that is soil solution. Because of these soluble salt/s, the concentration of soil solution increases.
If there is a lot of soluble salts in the soil solution, the difference of osmotic potential between soil water and plant cell get reduced gradually. As a result, it becomes much more difficult for the plant roots to absorb water from the soil.
In such a situation, plant expend energy to make osmotic adjustment that helps it to survive in such harsh condition. But the lost energy results in reduced growth of plants.
Osmotic adjustment
It is a special type of biochemical mechanism that helps plants to acclimatize to dry and saline conditions. Osmotic adjustment is the change in the solute substances (those are osmotically active) in the plant cell/s. It increases the concentration as well lower the osmotic potential of the plant cells.
Osmotic adjustment is achieved by accumulating substances including organic acids, inorganic ions (both cation and anions), carbohydrates and amino acids.
ii. Salinity injury
Salinity may delay or even prevent the germination of seeds. As young root cells encounter a soil solution high in salts, they may lose water by osmosis to the more concentrated soil solution. As a result-
- Plasmolysis of cells occurs.
- The cells get collapsed.
- Metabolic activities are hampered.
- The plant is destined to death.
Leaf shrinkage in dehydration (microscopic)
That is why there is a limit to the concentration of soil solution that can be tolerated by plants. The concentration in normal fertile soils varies from 0.05-0.2 % (expressed on the weight of dry soil).
6. Removes toxic substances-
When the soil solution is more dilute, the toxic substances are leached out from the soil through downward movement and thus the concentration of these toxic substances in the soil solution get reduced.
7. Controls the temperature of soil-
Water has a higher capacity to retain heat. At 0°C temperature, plant generally cannot uptake nutrients.
Pedological Importance of Soil Solution
1. Acts as an agent of weathering-
The most common weathering process of soil is hydrolysis and it is influenced by the soil water. In hydrolysis, water molecules split into their hydrogen and hydroxyl components and the hydrogen often replaces a cation from the mineral structure. A simple example is the action of water on Orthoclase (KAlSi3O8), a potassium containing feldspar.
The potassium released is soluble and is subject to adsorption by soil colloids, uptake by plants and removal in the drainage or leaching water.
The dissolved CO2 in soil solution reacts with water and form H2CO3 acid. Weathering is accelerated by the presence of acids, which increase the activity of hydrogen ions (H+). The carbonic acid (H2CO3) hastens the chemical dissolution of calcite in limestone or marble and releases calcium ion (Ca2+).
2. Soluble salts of soil solution control the properties of saline soil.
3. Soil solution (salt movement) controls the genesis of soil (e.g. saline soil)-
The deposition of particles depend on the movement of salts. In arid regions where
precipitation is less than evaporation, salts accumulate in soil, get adsorbed on soil colloids, and affect pH.
4. Controls the oxidation and reduction reaction occurring in soil-
When rocks containing minerals of Fe, Mn and S are exposed to soil water or soil solutions during soil formation, the iron is easily oxidized (loses an electron) and becomes trivalent Fe(III).
Source:
1. Class note
2. Osmotic Adjustment Under Drought Condition.
Authors: Gregor J. Sanders & Stefan K. Arndt.
 Plantlet The Blogging Platform of Department of Botany, University of Dhaka
Plantlet The Blogging Platform of Department of Botany, University of Dhaka






It was helpful.
Thank you.