The nitrogen cycle is the biogeochemical cycle by which nitrogen is converted into multiple chemical forms as it circulates among the atmosphere, terrestrial, and marine ecosystems. The conversion of nitrogen can be carried out through both biological and physical processes. Important processes in the nitrogen cycle include fixation, ammonification, nitrification, and denitrification. 1
Source of Nitrogen
Nitrogen, which has stable valence states ranging from -3, as in ammonia (NH₃) to +5, as in nitrate (NO3-), occurs in numerous oxidation states. Nitrogen is a constituent of amino acids, nucleic acids, amino sugars, and their polymers. A large, slowly cycled reservoir for nitrogen (3.8 x 1015 metric tons) is the nitrogen gas of the atmosphere (79%). Large but essentially unavailable reservoirs of nitrogen are present in igneous (1.4 x 1016 metric tons) and sedimentary (4.0 x 1015 metric tons) rock as bound, non exchangeable ammonia. Physiochemical and biological weathering releases ammonia from the reservoirs so slowly that it has little influence on early cycling.
The inorganic nitrogen ions such as ammonium, nitrite, and nitrate occur as salts that are highly water soluble and consequently are distributed in dilute aqueous solution throughout the ecosphere. They form small, actively cycled reservoirs. Living and dead organic matter also provides relatively small, actively cycled reservoirs of nitrogen.
Nitrogen cycle
Plants, animals, and most microorganisms require combined forms of nitrogen for incorporation into cellular biomass, but the little ability to fix atmospheric nitrogen is restricted to a limited number of bacteria, archaea, and symbiotic associations. The biogeochemical cycling of the element nitrogen is highly dependent on the activities of microorganisms.
Best safe and secure cloud storage with password protection
Get Envato Elements, Prime Video, Hotstar and Netflix For Free
Best Money Earning Website 100$ Day
#1 Top ranking article submission website
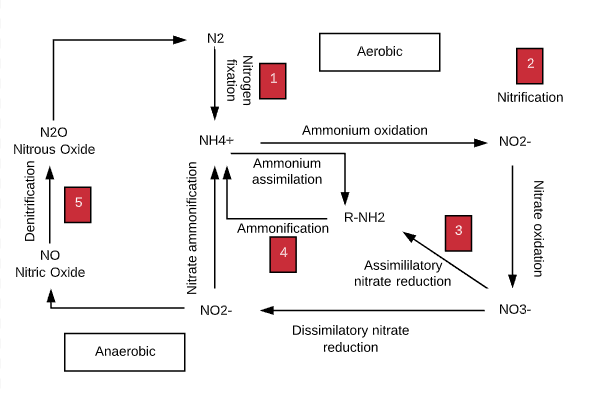
Ammonification
The release of ammonia from a simple nitrogenous organic compound, urea, can be described as follows.
- NH2CONH2 + H2O = 2NH3 + CO2
- Urease enzyme helps.
Nitrification
In nitrification, ammonia or ammonium ions are oxidized to nitrite ions (first equation) and then to nitrate ions (next equation).
- NH4+ + 1.5 O2 = NO2– + 2H+ + H2O
- NO2– + 1.5 O2 = NO3–
Denitrification
Denitrifying bacteria such as Paracoccus denitrificans, ThiobacilLus denitrificans, various Pseudomonads, Bacillus etc. converting NO3– through NO2 to nitric oxide (NO), and nitrous oxide (N2O) to molecular nitrogen. The denitrification sequence is as follows:
- NO3– → NO2– → NO → N2O → N
Revised by
- Md. Siddiq Hasan on 23 march, 2021
 Plantlet The Blogging Platform of Department of Botany, University of Dhaka
Plantlet The Blogging Platform of Department of Botany, University of Dhaka