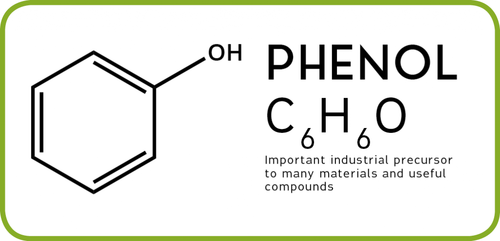
What is Phenol?
A large number of phenolic compounds which occur in plants as secondary metabolites are commonly known as Phenols. They are also known as Phenolies, Phenic acid, Benzenol and Carbolic acid.These compounds were first identified by German chemist Friedlieb Ferdinand Runge.
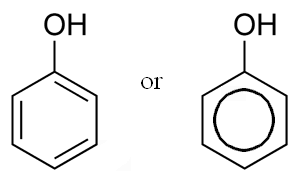
They all are a chemical compounds consisting of a hydroxyl group (-OH) bonded directly to an aromatic hydrocarbon group. Phenol was first extracted from coal tar but today is produced on a large scale (about 70 billion tones/year). The chemical formula of phenol is C6H5OH.
Characteristics of Phenol
- It Contains 6-10C atoms.
- They have low molecular weight.
- They are extremely diverse in chemical structure.
- Some are soluble in organic solvents and some are soluble in water while others may be large complex polymers that are insoluble.
- They are polar because of the hydroxyl group.
- Their polar side and hydrogen bonding allow them to dissolve in water.
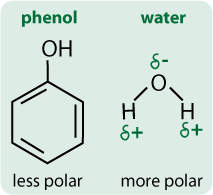
- Their non-polar side allow them to dissolve in certain organic solvents.
- They are acidic and toxic.
Classification of Phenol
Phenols are mainly divided into four groups –
- Simple phenols
- Phenolic carboxylic acids (or phenolic acids)
- Phenyl propane derivatives (or propenyl phenol)
- Flavan derivatives
I. Simple Phenols
They are aromatic organic compounds containing single benzene ring. e. g. hydro quinone, arbutin etc.
Best safe and secure cloud storage with password protection
Get Envato Elements, Prime Video, Hotstar and Netflix For Free
Best Money Earning Website 100$ Day
#1 Top ranking article submission website
II. Phenolic Carboxylic Acids
They are a type of organic compounds containing a phenolic ring and an organic carboxylic acid function. e. g. benzoic acid, gallic acid, vanilic acid etc.
III. Phenyl Propane Derivatives
They are a type of polyphenols. These compounds are the primary constituents of various essential oils. e. g. cinnamic acid, coumarin etc.
IV. Flavan Derivatives
They are the largest and best studied natural phenols which include several thousand compounds. e. g. flavan, catching, flavonol etc.
Uses of Phenol
A. Biological functions:
- Some of them act as chemical deterrents against herbivores and pathogens.
- Plant phenolics such as lignins provide mechanical strength to the plants and have significant protective functions in them.
- Some of them play important role in plants in attracting pollinators, fruits and seed disperses.
- Some plant phenolics play important role in Allelopathy (Greek allelon= of one another and pathos= diseases)
- Good to Know : Allelopathy is the influence of chemicals released by one plant species on another plants or animals with resulting benefits to the species which contains them.
B. Industrial Uses:
- The major uses of phenol, consuming two thirds of its production involve it’s conversion to plastics or related materials.
- It is also a versatile precursor to a large collection of drugs most notably aspirin but also many herbicides pharmaceutical drugs.
- They are used as an oral anesthetic or analgesic.
- They are also used in the preparation of cosmetics including sunscreens, hairdyes and skin lightening preparations.
- They are deficient in household cleaners.
References & Other Links
- Class Lecture of Prof. Dr. Rifat Samad mam, Department of Botany, University of Dhaka.
Written by
Tubaia Zannat Juthi, B.S. (Hons), Department of Botany, University of Dhaka
 Plantlet The Blogging Platform of Department of Botany, University of Dhaka
Plantlet The Blogging Platform of Department of Botany, University of Dhaka