The ER is the most extended organelle in plenty of eukaryotic cells: on average it occupies 10% of the cellular volume (Eelco and Roberto, 2016). The endoplasmic reticulum (ER) is a large, continuous organelle that extends throughout the entire cell (Eelco and Roberto, 2016). The ER not only is the medium of manufacture of maximum lipids and of membrane and secreted proteins, however also is the predominant Ca2+ storage in the cell and a hub for signal integration (Eelco and Roberto, 2016). Individual subregions of the ER have specialized tasks (Eelco and Roberto, 2016). In particular, contact sites with other organelles, for instance, mitochondria, are fundamental for signaling and lipid transfer (Eelco and Roberto, 2016). Through the unfolded protein response, the size and functional capacity of the ER are adapted in order to meet physiological and developmental requirements. (Eelco and Roberto, 2016).
Discovery of the Endoplasmic Reticulum
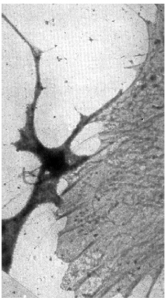
In 1945, a lacelike reticulum in cultured chick embryo cells was first observed by Keith Porter, Albert Claude, and Ernest Fullam. They made use of cultured cells because they were thin enough to be penetrated by an electron beam. This was significant since the ultramicrotome had not yet been invented. Just think about their excitement when they saw this beautiful lacelike structure revealed by the electron microscope.
Approximately 100 years earlier, Félix Dujardin had narrated protoplasm viewed with a light microscope as a substance which has “absolutely no trace of any organization, neither fibres, nor membranes, nor any sign of cellular structure.” Albert Claude separated it using the method of differential centrifugation which was initiated by Bensley and Hoerr (1934) following the discovery of the lacelike reticulum. For the first time, morphology and biochemistry could be together. Claude called the membranes as microsomes. Johannes Hanstein actually coined the term microsome to mean the unidentified vesicles noticed in plant cells. Claude used microsome as a noncommittal term asserting only the size. Claude chemically analyzed the microsomes and found that they contained approximately 9 % N, 2.5 % P, 40–45 % lipid, 0.75 % S, 0.01 % Cu, and 0.03 % Fe.
A few years later, the lacelike reticulum seen in the electron microscope was renamed the endoplasmic reticulum by Porter and Thompson (1948). With the arrival of the ultramicrotome and methacrylate embedding procedures, the ER was first observed with high resolution by George Palade and Keith Porter in 1954. With thin sections, it was possible to observe that the ER was composed of membranes that were 5.5 to 6.5 nm thick.
Perhaps it was fortunate that the lacelike reticulum was discovered before the invention of the ultramicrotome because it is possible that the three-dimensional arrangement of the endoplasmic reticulum may not have been found from 20 to 40 nm thick sections (Palade, 1956). In 1956, Palade, in addition to Siekevitz, started a study uniting electron microscopy and biochemistry, a combination that causes the award of the Nobel Prize to Palade (1975). Buvat and Carasso (1957) contributed a lot to the notion that the ER becomes a fundamental part of the protoplasm of eukaryotic cells by showing that it is present in the cells of the plant kingdom side by side those of the animal kingdom.
Physical Structure
The architecture of the ER is dynamic; it varies from cell to cell and changes throughout the cell cycle.
-
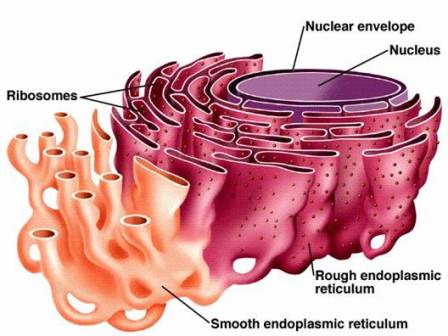
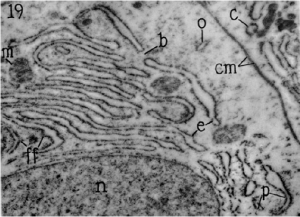
Structure of Endoplasmic Reticulum. Source: Biology Exams 4u. The endoplasmic reticulum (ER) is composed of two parallel membranes with an intervening lumen.
- The ER remains both as tubules and as lamellae, and some of the lamellae may have pores or fenestrations that are reminiscent of nuclear pores.
- Focal arrays of ER can also be seen and these may be sites of active membrane growth. Zheng and Staehelin (2001) call similar focal arrays nodal ER and suggest that the nodal ER in columella cells are engaged in gravity.
- Many kinds of enzymes are found in the membranes of the endoplasmic reticulum that are required for various important synthetic activities. The most significant enzymes are the stearases, NADH-cytochrome C reductase, NADH diaphorase, glucose-6-phosphatase, and Mg++ activated ATPase.
- The membrane of endoplasmic reticulum remains connected with the membranes of the plasma membrane, nuclear membrane, and Golgi apparatus.
- The cavity of the endoplasmic reticulum is developed thoroughly and works as a passage for the secretory products.
The ER comprises three types of elements:
The Cisternae
- RER remains as loosely packed stacks of cisternae.
- Cisternae are thin, flat, variably sized which are connected via means of thin tubular elements.
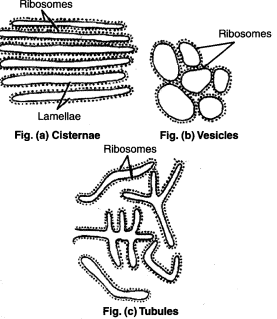
Elements of Endoplasmic Reticulum. Source: Discourse. - The cisternae of the endoplasmic reticulum are connected with both the perinuclear cisternae and the extracellular space.
The Vesicles
- They are often seen isolated in the cytoplasm and occur in most cells but especially sufficient within the SER.
- In few places, in particular, at their ends, the cisternae are swelled into shapes corresponding to vacuoles, balloons, or flasks. Those vacuolar structure is vesicle.
- Vesicles bleb off from the transition ER and conjoin with the Golgi apparatus.
The Tubules
- Tubules can be highly curved and branched to make a polygonal network extending throughout the entire cytosol.(Eelco and Roberto, 2016)
- ER tubules are cylindrical with a diameter of 30–100 nm (Eelco and Roberto, 2016)
- The tubules (both rough and smooth) form a continuous membrane system that has the nuclear envelope (Voeltz and Prinz, 2007).
- Tubules are usually found in RER.
Types of Endoplasmic Reticulum
Two sorts of ER exist:
The granular or rough form (RER) and the agranular or smooth form (SER). These granules are about 150Å in diameter and have been identified as membrane-bound ribosomes.
1. Rough Endoplasmic Reticulum
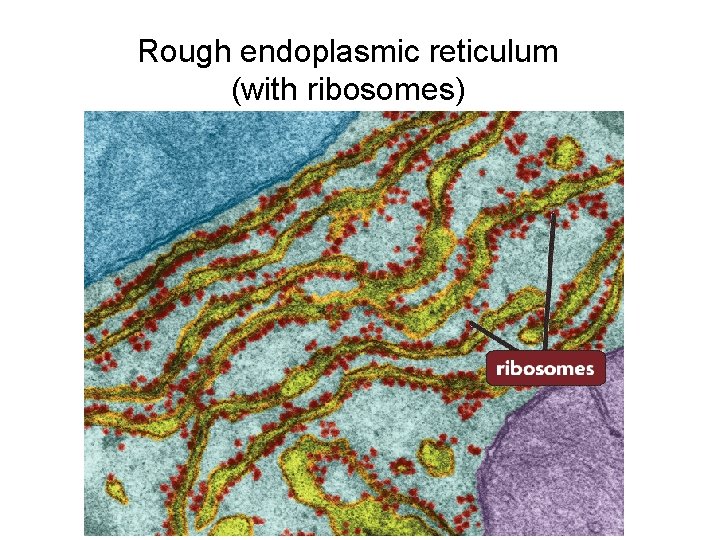
Elaborate systems of RER membranes are located predominantly in cells. The RER is “studded” with ribosomes. On the cytoplasmic surface of the endoplasmic reticulum, there are polyribosomes, giving them a granular appearance. The rough endoplasmic reticulum composes of tubules and flattened cisterns. The RER is attached to the nuclear envelope at one end and is continuous with the SER at the other end.
Function of RER
- Protein synthesis on the endoplasmic reticulum: Special proteins have been synthesized in the RER of animal cells that bind the large subunit of the ribosome and prevent the lateral movement of the ribosome to the SER. The RER has approximately 20 extra sorts of polypeptides than the SER. Some of those polypeptides may be involved in ribosome anchoring; others may be involved in keeping the shape of the flattened cisternae.
- Protein secretion: Rough (granular) endoplasmic reticulum (RER) is remarkable in cells specialized for protein secretion, along with pancreatic acinar cells (digestive enzymes), fibroblasts (collagen), plasma cells (immunoglobulin).
- Smooth ER formation: Rough ER proliferates to smooth ER, in which the synthesis of lipid and steroid molecules occurs, cholesterol among others.
- Protein Glycosylation (Carbohydrate Synthesis): Protein glycosylation takes place in the ER where a single type of oligosaccharide is added to amino group of asparagine.
- Formation of nuclear envelope: The RER forms nuclear envelope around daughter cells during cell divisions.
2. Smooth Endoplasmic Reticulum
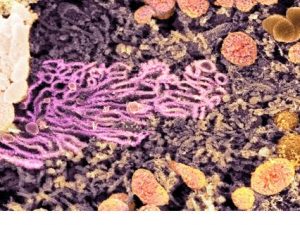
The smooth (agranular) endoplasmic reticulum (SER) is the membranous network inside the cell. These are devoid of ribosome granules. Its cisterns are more tubular and much more likely to seem as a profusion of interconnected channels of variable sizes and shapes. It usually becomes the form of a tightly woven network of branched tubules of various diameters. Cisternae are usually absent.
Function of SER
- Lipid Synthesis: They are concerned with steroid synthesis, lipid metabolism, and oil bodies which are needed for membrane synthesis.
- SER mediated cell-to-cell communication via plasmodesmata and detoxication processes.
- SER metabolizes many xenobiotic substances, along with pharmaceuticals, pesticides, and carcinogens, etc. The SER is therefore the most important intracellular detoxification process.
- Smooth ER is known as sarcoplasmic reticulum in striated skeletal muscle tissue, wherein it serves to store calcium.
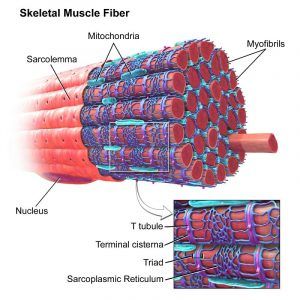
a skeletal muscle, with the sarcoplasmic reticulum colored blue. Source: Biology Dictionary.
The transition between granular and agranular ER may be continuous.
Others Function of Endoplasmic Reticulum
ER is composed of a couple of different structural domains, each of which is associated with a specific function or functions.
- Inhibitors: The traditional inhibitors of protein synthesis are puromycin, chloramphenicol, cycloheximide, and streptomycin. Chloramphenicol inhibits chloroplast protein synthesis however not cytoplasmic protein synthesis.
- In vitro protein synthesis: Protein synthesis can occur in vitro by supplying reagents for synthesis. Such systems offer a tool for investigating the components for protein synthesis.
- Ubiquitylation: This process is significant in the degradation of misfolded proteins. Ubiquitin is anchored in the ER membrane. Ubiquitylation performs a function in protein degradation through the proteasome.
- Also, the plant ER can accumulate hydrolytic enzymes within ER in response to pathogens.
- Calcium regulation: the ER can function as a storage site for Ca2+ and is involved with Ca2+ Calcium is a widespread signaling molecule that could have an effect on numerous processes including localization, function, and association of proteins, either with other proteins, organelles, or nucleic acids. Calcium may also play a function in reshaping the ER in response to cellular signals.
- Phenylpropanoid and Flavonoid synthesis: The phenylpropanoid pathway is a part of the plant aromatic pathway, and one of the principal enzymes of this pathway is embedded within the ER as a segment of a multienzyme complex referred to as a metabolon.
- Auxin homeostasis: Another function of ER is auxin homeostasis. This is accomplished by PIN-Formed (PIN) proteins.
- Finally, the ER can house monooxygenases that can convert ent-kaurene to GA122, a precursor of the family of gibberellins.
- The ER functions in maintaining a surface-to-volume ratio in large cells of about 106m-1 and thus duplicates many of the transport functions of the plasma membrane.
- The ER additionally works as the workbench of the cell for building itself and other membranes and accordingly is endowed with the enzymes necessary for lipid synthesis and ribosomes for protein synthesis. So proteins that are synthesized on the ER go there because they contain a signal peptide. The signal hypothesis narrates a general mechanism of how specific proteins are targeted to each organelle.
Biogenesis
The mechanisms responsible for ER formation and morphology are opaque. In yeasts, ER tubules, microtubules, and molecular motors are vital. The motors appear to be F‐actin and myosins. ER exit sites (ERES) are significant in protein export. The molecular mechanisms for their biogenesis and maintenance are not completely understood. Considerable research is needed to set up the precise events underlying ER biogenesis.
Ribosome synthesis involves processing of a polycistronic pre‐ribosomal RNA to the mature rRNA of the 40S subunit and the 5.8S/28S rRNA of the 60S subunit. RNA polymerase I drives the synthesis, cleavage, and modification of pre‐RNA in eukaryotes; ribosome assembly requires about 200 essential factors. Chen et al. (2011) point out that the ER network can rearrange in response to developmental signs. This rearrangement comprises continuous fusion and fission reaction. The shaping of the cell tubular end appears to be mediated via way of means of the membrane proteins, reticulons, and atlastin.
References
- Color Atlas of Cytology, Histology and Microscopic Anatomy by Wolfgang Kuehnel.
- Eelco van Anken, Roberto Sitia, 2016. The Endoplasmic Reticulum, 157-158.
- Histology, Cytology, Embryology Textbook by Bobrysheva I. V. & Kachshenko S. A.
- Plant Cell Biology from Astronomy to Zoology by Randy Wayne.
- Plant Cells and Their Organelles by William V. Dashek & Gurbachan S. Miglani.
- Voeltz, G.K., Prinz, W.A., 2007. Sheets, ribbons, and tubules – how organelles get their shape. Nature reviews. Molecular Cell Biology 8, 258–264.[1]
 Plantlet The Blogging Platform of Department of Botany, University of Dhaka
Plantlet The Blogging Platform of Department of Botany, University of Dhaka


Very much fascinated with your works. Such development within such a short period.
Go ahead.
Thank you..It wouldn’t be possible without help from plantlet..Keep supporting.
Lots of information about ER in one place. Beneficial indeed.
Thanks a ton…
So much detailed information! Great work.
Thank you so much
Something nice to read. So much informative..
Thanks a lot
I may need your help. I tried many ways but couldn’t solve it, but after reading your article, I think you have a way to help me. I’m looking forward for your reply. Thanks.
How can I help you?