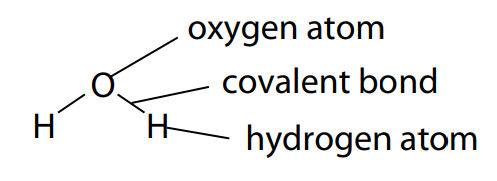
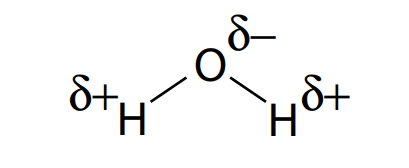
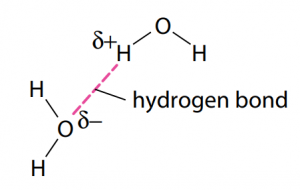
Carbohydrates are fundamentally the sugars, starches and fibers found in fruits, grains, vegetables and milk products. Though often it slander in trendy diets, carbohydrates which is one of the basic food groups which are important to a healthy diet. A carbohydrate is a biomolecule containing of carbon (C), hydrogen (H) and oxygen (O) atoms, normally with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the seen formula Cm(H2O)n (where m may be different from n). Additionally, not all carbohydrates match to this precise stoichiometric definition (e.g., uronic acids, deoxy-sugars such as fucose), nor are all chemicals that do match to this definition automatically classified as carbohydrate.
Carbohydrates are a form of macronutrients, meaning they are one of the three main ways in which the body obtains energy, or calories. The American Diabetes Association notes that carbohydrates which are the body’s main source of energy. They are called carbohydrates because of the chemical level, they contain carbon, hydrogen and oxygen.
Best safe and secure cloud storage with password protection
Get Envato Elements, Prime Video, Hotstar and Netflix For Free
Best Money Earning Website 100$ Day
#1 Top ranking article submission website
There are three macronutrients which are carbohydrates, protein and fats. Macronutrients are essential for the functioning of our body, and the body requires large quantities of them. All macronutrients should be obtained through diet; the human body cannot produce macronutrients on its own.
All carbohydrates contain the basic elements which are carbon, hydrogen and oxygen. The ‘hydrate’ part of the name comes from the fact in which hydrogen and oxygen atoms are present in the ratio of 2 : 1, as they are in water (‘hydrate’ refers to water). The general formula for a carbohydrate also be written as Cx(H2O)y.
Carbohydrates are divided into three main groups which in namely monosaccharides, disaccharides and polysaccharides. The word ‘saccharide’ which refers to a sugar or sweet substance.
Monosaccharides
Monosaccharides are basically sugars. Sugars dissolve in water easily to form sweet-tasting solutions. Monosaccharides have the universal formula (CH2O)n and consist of a single sugar molecule (‘mono’ means one). The main types of monosaccharides, if they are classified according to the number of carbon atoms in each and every molecule, are trioses (3C), pentoses (5C) and hexoses (6C). The names of all sugars end with -ose normally. Common hexoses which are glucose, fructose and galactose. Two common pentoses are the ribose and deoxyribose.
A macromolecule is a huge biological molecule such as a protein, polysaccharide or nucleic acid.
A monomer is a relatively simple molecule which is used as a basic building chunks for the synthesis of a polymer and many monomers are joined together in order to make the polymer, usually by condensation reactions and usual examples of molecules used such as monomers are monosaccharides, amino acids and nucleotides.
A polymer is a leviathan molecule made from many similar repeating subunits joined together in a chain and the subunits are much smaller and simpler molecules known as monomers. Examples of biological polymers which are are polysaccharides, proteins and nucleic acids.
Molecular and structural formulae
The formula for a hexose could be written as C6H12O6. This is familiar as the molecular formula. It is also useful to show the arrangements of the atoms and which can be done using a diagram known as the structural formula. Figure (A) shows the structural formula of glucose, a hexose, which is the most common monosaccharide.
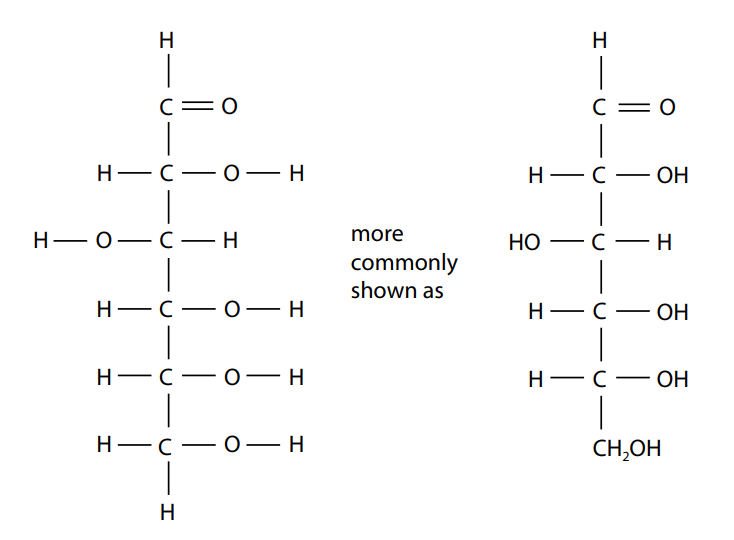
Ring structures
One important feature of the structure of pentoses and hexoses is that the chain of carbon atoms is long enough to shut up on itself and form a more stable ring structure. This can be ornamented using glucose as an example. Whenever glucose forms a ring, carbon atom number 1 joins to the oxygen on carbon atom number 5 (Figure B). The ring thus contains oxygen, and carbon atom number 6 is not a part of the ring.
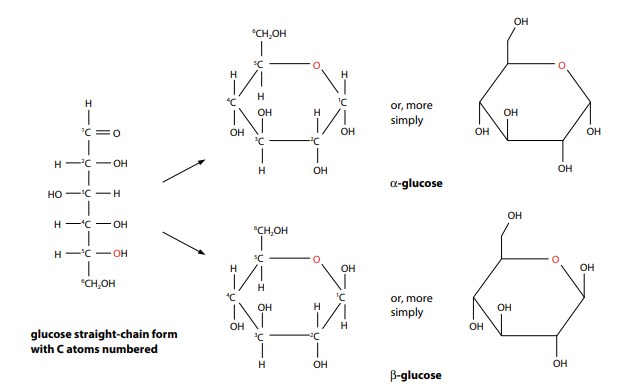
Roles of monosaccharides in living organisms
Monosaccharides has two major functions. First, they are regularly used as a source of energy in respiration. This is awaited to the large number of carbon–hydrogen bonds. These bonds can be burst to release a lot of energy and which is transferred to help make ATP (adenosine triphosphate) from ADP (adenosine diphosphate) and phosphate. The most necessary monosaccharide in energy metabolism is glucose.
Secondly, monosaccharides which are important as building blocks for larger molecules. For example, glucose which is used to make the polysaccharides starch, glycogen and cellulose. Ribose (a pentose sugar) is one of the molecules used to make RNA (ribonucleic acid) and ATP. Deoxyribose (also a pentose sugar) is one of the molecules used to make DNA).
Disaccharides and the glycosidic bond
Disaccharides which is like monosaccharides, are sugars. They are organized by two monosaccharides joining together. The three most usual disaccharides are maltose (glucose + glucose), sucrose (glucose + fructose) and lactose (glucose + galactose). Sucrose is the transport sugar in plants and that sugar usually bought in shops. Lactose is the sugar found in milk and is therefore an important essential of the diet of young mammals.
The joining of two monosaccharides takes place by a process which is known as condensation. Two examples are displayed in Figure (C) In Figure (C1) two molecules of α-glucose combine to make the disaccharide maltose. In Figure (C2) α-glucose and β-fructose mixed in order to make the disaccharide sucrose. Notice that fructose has a unlike ring structure to glucose.
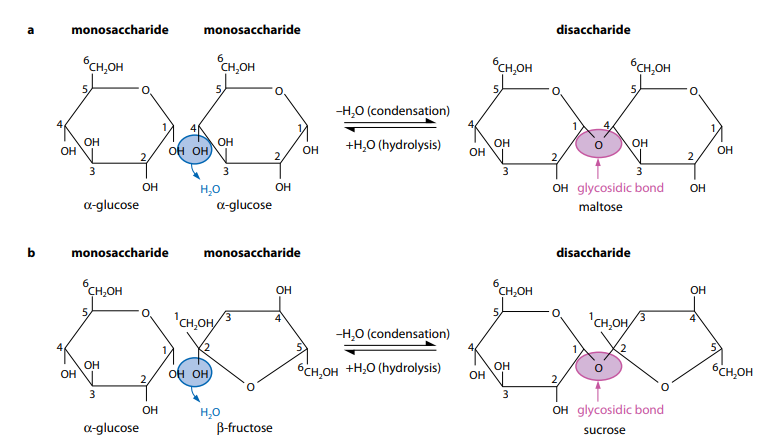
A monosaccharide is a molecule be made up of a single sugar unit with the general formula (CH2O)n.
A disaccharide is a sugar molecule be made up of two monosaccharides joined together by a glycosidic bond.
A polysaccharide is a polymer whose subunits are monosaccharides connected together by glycosidic bonds.
For each and every condensation reaction, two hydroxyl (–OH) groups line up alongside each other. One combines with a hydrogen atom from the other to form a water molecule and this allows an oxygen ‘bridge’ to form between the two molecules, bonding them together and forming a disaccharide (‘di’ means two). The bridge is been called a glycosidic bond.
In premise any two –OH groups can line up and, since monosaccharides have many –OH groups, there are a huge number of possible disaccharides. The size of the enzyme controlling the reaction determines which –OH groups come alongside each other. Only a little of the possible disaccharides are common in nature.
The alter of condensation is the addition of water, which is known as hydrolysis (Figure C). This takes place during the digestion of disaccharides and polysaccharides and when they are broken down to monosaccharides.
Polysaccharides
Polysaccharides are polymers and it’s subunits (monomers) are monosaccharides. They are made by connected many monosaccharide molecules by condensation. Each and every successive monosaccharide is added by means of a glycosidic bond, as in disaccharides. The last molecule may be several thousand monosaccharide units long, forming a macromolecule. The most main polysaccharides are starch, glycogen and cellulose, all of which are polymers of glucose. Polysaccharides are no sugars.
Since glucose is the primary source of energy for cells, it is important for living organisms to reserve it in an appropriate form. If glucose itself accumulated in cells, it would dissolve and make the contents of the cell too concentrated, which would seriously modify its osmotic properties. Glucose itself also a reactive molecule and would interfere with normal cell chemistry. These troubles are avoided by converting glucose, by condensation reactions, to a storage polysaccharide, which is a convenient, compact, inert (unreactive) and insoluble molecule. The amount of storage polysaccharide formed is starch in plants and glycogen in animals. Glucose could be made available again quickly by an enzyme-controlled reaction.
Starch and glycogen
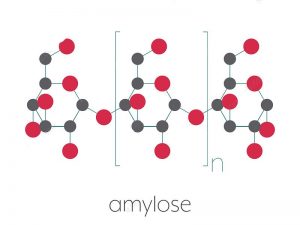
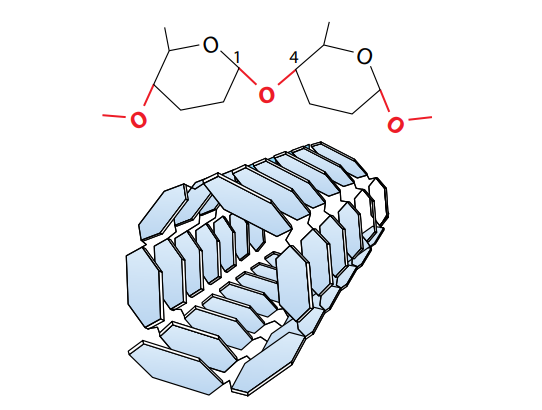
Starch is a fusion of two substances – amylose and amylopectin. Amylose is made by condensations in the middle of α-glucose molecules, as shown in Figure (F)a. In this way, a extended, unbranching chain of several thousand 1,4 linked glucose molecules is built up. (‘1,4 linked’ means they are linked in the middle of carbon atoms 1 and 4 of successive glucose units.) The chains are curved (Figure D) and coil up into helical structures just like springs, making the final molecule more compact. Amylopectin which is also made of many 1,4 linked α-glucose molecules, but the chains are shorter than in amylose, and branch out to the sides. The branches are formed by 1,6 linkages, as shown in Figure (F).
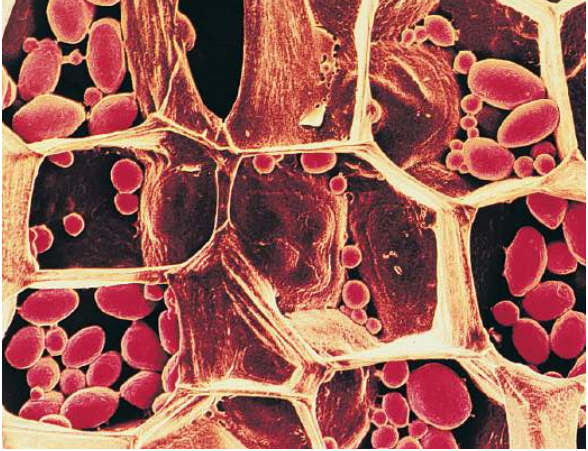
Many of amylose and amylopectin molecules assemble up into relatively large starch grains, which are commonly found in chloroplasts and in storage organs such as potato tubers and the seeds of cereals and legumes (Figure E). Starch grains which are easily seen with a light microscope, mainly if stained and rubbing a freshly cut potato tuber on a glass slide and staining with iodine–potassium iodide solution which is a quick method of producing a specimen for broadcasting.
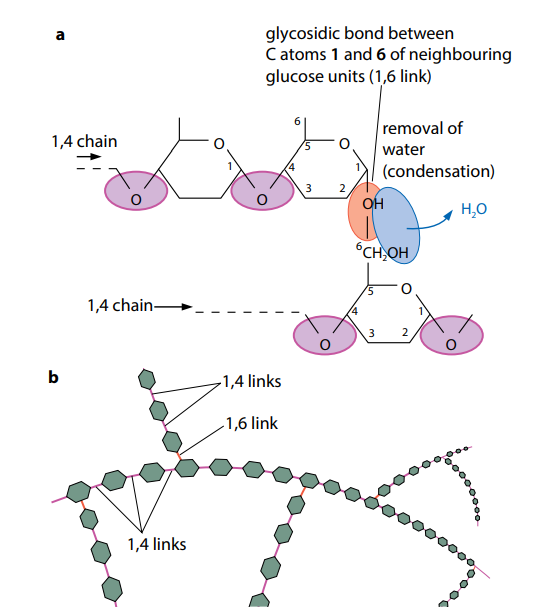
Starch is nowhere found in animal cells. Rather, a substance with molecules very like those of amylopectin is used as the storage carbohydrate. This is called “Glycogen“. Glycogen which is like amylopectin, is made of chains of 1,4 linked α-glucose with 1,6 linkages forming branches (Figure Fb). Glycogen molecules lean to be even more branched than amylopectin molecules. Glycogen molecules brunch together to form granules, which are visible in liver cells and muscle cells, where they form an energy reserve.
Cellulose
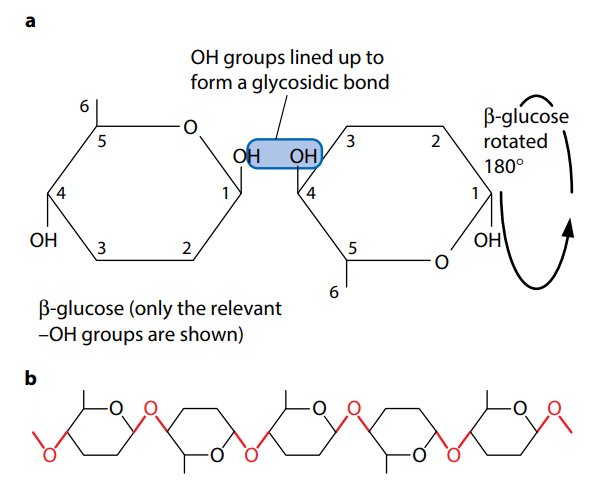
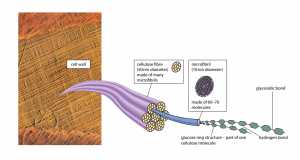
Cellulose is the most bountiful organic molecule on the planet, due to its presence in plant cell walls and its slow rate of breakdown in nature. It has a structural role, being a mechanically powerful molecule, unlike starch and glycogen. Though, the only difference between cellulose and starch and glycogen is that cellulose is a polymer of β-glucose, not α-glucose.
The groundwork of β-glucose molecules results in a strong molecule because the hydrogen atoms of –OH groups are weakly attracted to oxygen atoms in the same cellulose molecule (the oxygen of the glucose ring) and also to oxygen atoms of –OH groups in neighboring molecules. These hydrogen bonds are separately weak, but so many can form, due to the large number of –OH groups, that collectively they provide enormous strength. In the middle of 60 and 70 cellulose molecules turn out to be tightly cross-linked in order to form bundles called microfibrils. Microfibrils are in turn grasp together in bundles called fibers by hydrogen bonding. Thus successive glucose units are linked at 180° to each other, as shown in Figure H.
A cell wall typically has several layers of fibers, running in different directions in order to increase strength (Figure I). Cellulose makes up about 22–45% of the average cell wall and other molecules help to cross-link the cellulose fibers, and some form a glue-like matrix around the fibers, which increases more strength.
Cellulose fibers had a very big tensile strength, almost equal to that of steel. That means if pulled at both ends they are very difficult to stretch or break, and makes it possible for a cell to withstand the big pressures that develops within it as a result of osmosis. Without the wall, the cell would blowout when in a dilute solution. These pressures aids provide support for the plant by making tissues rigid, and are responsible for cell enlargement during growth. The arrangement of fibers around the cell aids to determine the shape of the cell as it grows.
 Plantlet The Blogging Platform of Department of Botany, University of Dhaka
Plantlet The Blogging Platform of Department of Botany, University of Dhaka