Nitrogen fixation is a process by which molecular nitrogen in the air is converted into ammonia (NH3) or related nitrogenous compounds in soil. Atmospheric nitrogen is a relatively nonreactive molecule that is metabolically useless to all but a few microorganisms. Biological nitrogen fixation converts N2 into ammonia, which is metabolized by most organisms.
Nitrogen fixation is essential to life because fixed inorganic nitrogen compounds are required for the biosynthesis of all nitrogen-containing organic compounds in organism’s body, such as amino acids and proteins, nucleoside triphosphates and nucleic acids (DNA & RNA).
Nitrogen fixation is carried out naturally in soil by microorganisms termed diazotrophs that include bacteria such as Azotobacter and archaea. Some nitrogen-fixing bacteria have symbiotic relationships with plant groups, especially legumes or bean plants.
Symbiotic Nitrogen Fixation
Bacterial Nitrogen Fixation
- Convert N2 gas to NH4+ (ammonium).
- Symbiotic N2 fixing bacteria live in the plant root nodules (anaerobic) in poor N2 conditions.
- Bacteria (e.g. leguminous, Rhizobia – symbiotic, cyanobacteria – free living) give plants with fixed N2 and plants give bacteria with nutrients and carbs (carbohydrates).
The nitrogen in the atmosphere is fixed by nitrogenase enzyme produced by the bacteria. But these enzymes are very oxygen sensitive. They require an oxygen free environment (anaerobic) to function. But again, the bacteria responsible for nitrogen fixation are aerobic. This paradox is solved by nodule formation.
Nodules provide anaerobic conditions by
- Low gas permeability
- Leghemoglobin (structure below)
Leghemoglobin is an oxygen-carrying protein, somewhat similar to the hemoglobin of blood, that helps maintain the low oxygen tension necessary for effective nitrogen fixation. The main functions of leghemoglobin are (1) to facilitate oxygen supply to the nitrogen fixing bacteria and (2) to protect the enzyme, nitrogenase from being inactivated by oxygen. (Source here)
Plant gives
- Nodule, nutrients.
- Globin part of leghemoglobin that helps in oxygen transport.
Bacteria give
- Fixed N2 via Nitrogenase enzyme.
- Heme part of hemoglobin.
Best safe and secure cloud storage with password protection
Get Envato Elements, Prime Video, Hotstar and Netflix For Free
Best Money Earning Website 100$ Day
#1 Top ranking article submission website
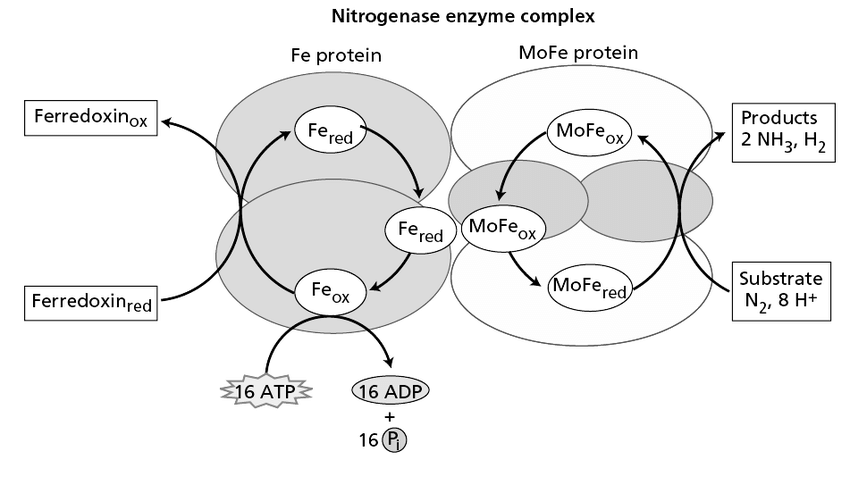
Nitrogenase reaction
12 + 8 Fd red + 8 H+ + 10 ATP → 2NH3 + H2 + 16 ADP + 16 Pi
By redox reaction in Fe protein and reducing MoFe (iron-molybdenun) protein. (Fd red means reduced ferredoxin (?)).
Non Symbiotic Nitrogen Fixation Diazotrophe
Microorganisms that pass independent life and fix atmospheric nitrogen are known as free-living diazotrophs. There are two groups of such microorganisms: bacteria and cyanobacteria (blue-green algae). Based on the mode of nutrition (carbon, nitrogen and oxygen and requirement of reducing groups) bacteria are divided into:
- Aerobic bacteria: Azonomas, Azotobacter Beijerinckia, Mycobacterium, Methylomonas.
- Facultative anaerobic bacteria: Bacillus, Enterobacter, Klebsiella, etc.
- Anaerobic bacteria: Clostridium.
- Photosynthetic bacteria: Rhodomicrobium, Rhodopseudomonas, Rhodospirillum, Chromatium, Chlorobium etc.
Among cyanobacteria both heterocystous and non-heterocystous forms fix atmospheric nitrogen, for example, Anabaena, Anabaenopsis, Aulosira, Calothrix, Cylindrospermum, Gloeocapsa, Lyngbya, Nostoc, Oscillatoria, Plectonema, Scytonema, Stegonema, Tolypothrix, Trichodesmium etc.. (Alexander, 1977).
Nitrogenase Enzyme
Nitrogenase is the enzyme complex responsible for nitrogen fixation. This has been characterized as two components, and neither is active without the other.
- Component I is dinitrogenase. It is known as MoFe protein.
- Component II is dinitrogenase reductase, known as Fe protein.
Nitrogenase is extremely sensitive to oxygen, requiring low oxygen tension for activity. This fixation of nitrogen needs not only nitrogenase, but also ATP, reduced ferredoxin.
Leghemoglobin
(also leghaemoglobin or legoglobin) is a nitrogen or oxygen carrier, because naturally occurring oxygen and nitrogen interact similarly with this protein, and a hemoprotein found in the nitrogen-fixing root nodules of leguminous plants. It is produced by legumes in response to the roots being infected by nitrogen-fixing bacteria, termed Rhizobia, as part of the symbiotic interaction between plant and bacterium; roots uninfected with Rhizobium do not synthesize leghemoglobin.
Good to know:
- Holoprotein is the whole leghemoglobin i.e both heme and globin part.
- Heme part is Fe and globin part is protein.
- The ‘globin’ part is also called apoprotein.
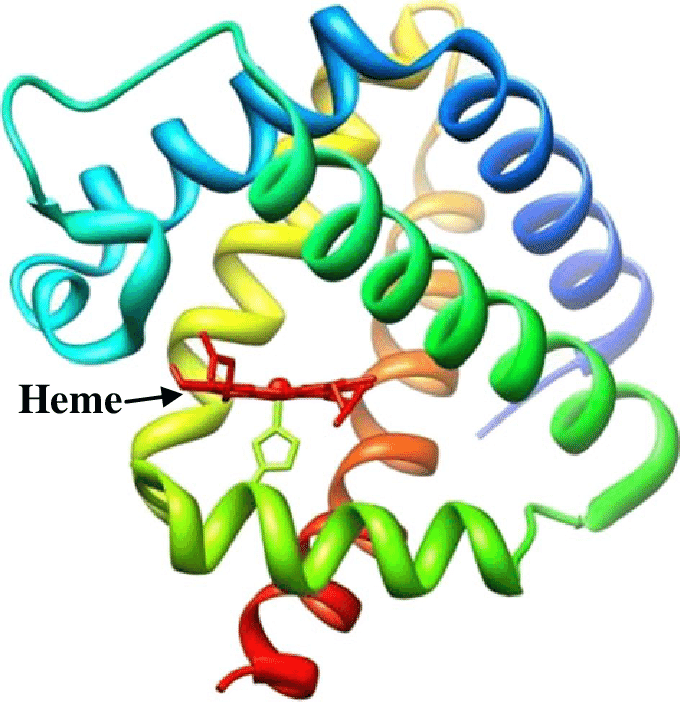
Structure of Leghemoglobin
Leghemoglobin has close chemical and structural similarities to hemoglobin, and, like hemoglobin, is red in color.
- The holoprotein (protein + heme cofactor) is widely believed to be a product of both plant and the bacterium in which the apoprotein (globin) is produced by the plant and the heme (an iron atom bound in a porphyrin ring) is produced by the bacterium.
- There is some evidence however suggesting that the heme portion is also produced by the plant.
In plants infected with Rhizobium, (such as alfalfa or soybeans), the presence of oxygen in the root nodules would reduce the activity of the oxygen-sensitive nitrogenase – an enzyme responsible for the fixation of atmospheric nitrogen. Leghemoglobin buffers the concentration of free oxygen in the cytoplasm of infected plant cells to ensure the proper function of root nodules.
Leghemoglobin has a high affinity for oxygen, about ten times higher than the chain of human hemoglobin. This allows an oxygen concentration that is low enough to allow nitrogenase to function but high enough so that it can provide the bacteria with oxygen for respiration.
Although leghemoglobin was once thought to provide a buffer for nodule oxygen, recent studies indicate that it stores only enough oxygen to support nodule respiration for a few seconds. Its function is to help provide oxygen to the respiring symbiotic bacterial cells in a manner analogous to hemoglobin transporting oxygen to respiring tissues in animals.
Nitrogen fixation in nodules
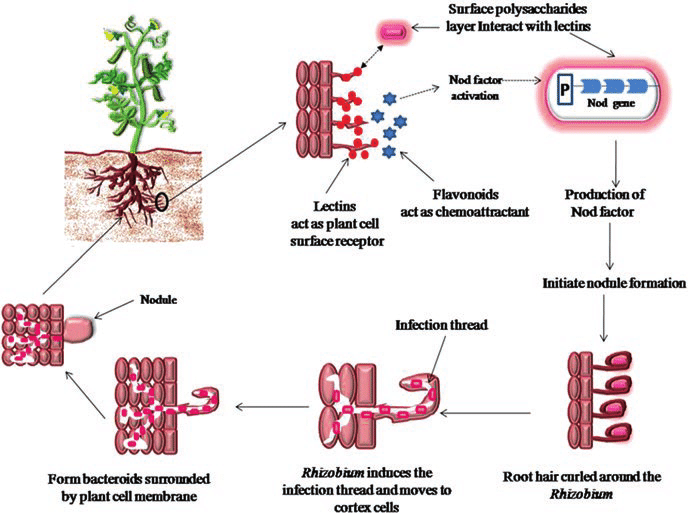
- One of the most important mutualistic relationships between microorganisms and plants involves the invasion of the roots of suitable host plants by nitrogen fixing bacteria resulting in formation on tumor like growth called a nodule.
- Within the nodule, the nitrogen fixing bacteria are able to convert atmospheric nitrogen to ammonia which supplies the nitrogen required for bacterial and plant growth.
- The fixation of nitrogen in plant nodules is of extreme importance for the maintenance of soil fertility in agricultural practices, it is used to increase crop yields.
- The fixation of atmospheric nitrogen depends on the nitrogenase enzyme system.
- Nitrogenase is very sensitive to oxygen and irreversibly inactivated on exposure to even low concentration. Nitrogen fixation, therefore, often is restricted to habitats in which nitrogenase is protected from exposure to molecular oxygen.
Nitrogen fixing associations between Rhizobia and Legumes
- The nitrogen-fixing associations of rhizobia with leguminous plants are of great importance both in global nitrogen cycling and in agriculture.
- Until recently, all nodulating and nitrogen fixing bacteria associated with leguminous plants were placed into a single genus, Rhizobium.
- Now two additional genera, Azorhizobium and Bradyrhizobium, are recognized.
- Rhizobium species are fast growing whereas Bradyrhizobium grows slowly (brady means slow).
- Bradyrhizobium species nodulate soybeans, lupins, cowpeas, and various tropical leguminous plants.
- Rhizobium species nodulate alfalfa, peas, clover, and a wide variety of other leguminous plants.
- Azorhizobium is a unique member of the group that forms stem nodules on tropical leguminous trees (Sesbania rostrata).
- In contrast to members of the two other genera, Azorhizobium is capable of growing with atmospheric nitrogen in its free living state. Rhizobium and Bradyrhizobium are not capable of doing so.
Azotobacter, for example, is a genus of free living bacteria that converts atmospheric nitrogen into ammonium, making it available for plant use. This process may only take place, however, if the following conditions are met:
- An easily degradable carbon source is available.
- Any nitrogen compounds such as ammonium or nitrate, are not already in present substantial concentrations.
- Soil pH levels are between 6 and 9.
- High levels of phosphorus are present.
- Very low levels of oxygen are present.
Revised by
- Md. Siddiq Hasan on 22 March, 2021
 Plantlet The Blogging Platform of Department of Botany, University of Dhaka
Plantlet The Blogging Platform of Department of Botany, University of Dhaka