Over four-fifths of the surface of our planet is covered with a salt solution (oceans & seas) containing, among many other constituents, approximately 0.5M NaCl. Still, only very few groups of higher plants can withstand such conditions. Most terrestrial species are unable to tolerate even one-tenth of the salt concentration of ocean water without a serious setback in their water and nutrient balance or in their metabolism.
Salinity is a common phenomenon and one of the basic features of arid and semiarid regions. Even though information regarding the natural processes leading toward salinization is essential for understanding the salt status in any habitat, the quest for the origin of such salts and their circulation in nature has been practically neglected. Results of only a few investigations dealing with such problems are available (Menchikovsky, 1924; Eriksson, 1958; Junge, 1958; Yaalon, 1963).
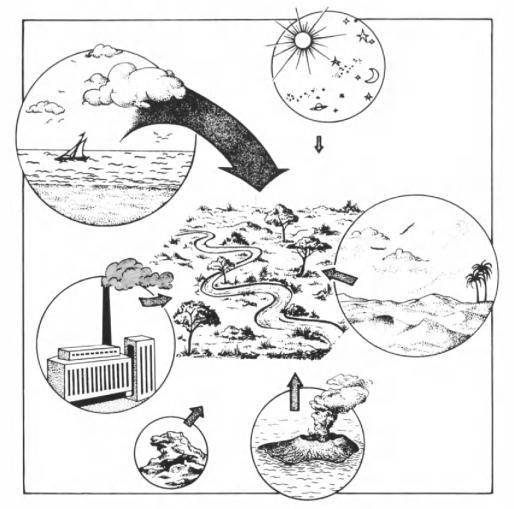
The major sources of salinity are classified as follows:
- Marine
- Lithogenic and
- Anthropogenic
Marine sources
Volcanic eruptions release large quantities of soluble salts into the atmosphere. It is generally accepted that vast amounts of chlorine and sulfur from this source have accumulated during the ages in the oceans, either directly by underwater volcanic action, precipitation of volcanic ash, dissolution of gases, or indirectly via streams and rivers which wash soluble salts off the continents.
However, salts were not left evenly distributed. After continuous cycling for long geological eras, distribution of various elements and compounds between different localities had distinctively occurred; while the more soluble salts of chlorine were leached and accumulated mostly in the oceans, over half of the compounds containing sulfur, presently found in the geochemical cycle, precipitated and concentrated in rocks sedimented under seawater. A small quantity of sulfur can also be found in igneous rocks.
Salts of marine origin are transported to the continents and deposited there in three major ways:
- Cyclic salts
- Infiltrating salts and
- Fossil salts
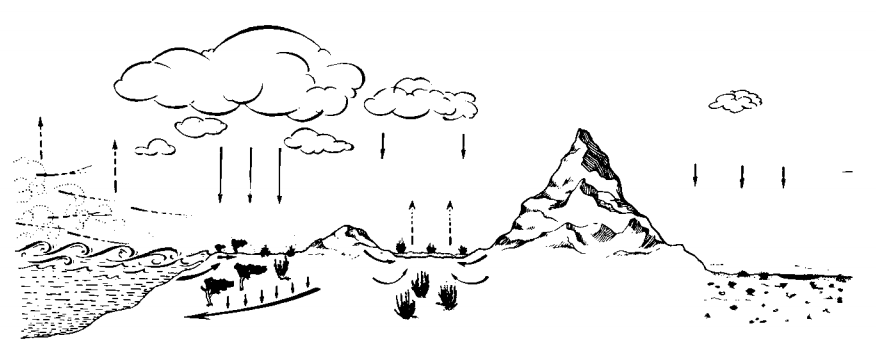
A) Cyclic salts: Salts brought inland from the sea in the form of windborne sea spray, and precipitated by rainwater. Such salts are later redistributed or leached back into the ocean by the common drainage systems.
B) Infiltrating salts: Salts brought into coastal habitats by an underground infiltration of seawater. This is a source of local importance.
C) Fossil salts: Marine salts which were precipitated sometime in the past in certain localities and which are now being dissolved. Such salts are brought above ground level or into the plant root zone by flowing masses of groundwater, by springs, by capillary movement of water, or by surface runoff water.
Lithogenic
Large differences exist in nature between distribution of chlorine and sulfur. Because of its high solubility, chlorine occurs in all rocks in very low quantities. In contrast, a large portion of the total amount of S released, in the past, into the oceans was precipitated into sedimentary rocks.
Low amounts of chloride and relatively high amounts of sulfur are found in rocks.
Chlorine is primarily found in rocks as chlorides of the alkali metals (e.g. NaCl). Among sedimentary rocks, sandstones show the lowest amounts of chloride. Limestones usually contain more chloride and more sulfur than shale.
S is primarily found in rocks in two chemical forms; in hard limestones it occurs as pyrite, and in soft limestones as sulfates, mostly gypsum.
The extent of salinization of groundwater and soils by chloride or sulfate from rock sources depends on the rate of rock weathering, which varies in different climates and regions. Under specific condition, weathering of saline rocks may also contribute large amounts of soluble salts.
Good to know
- Presumably, chloride is not incorporated into rock crystals, but is precipitated as intercrystal penetrations. In such a location and form, chlorides can migrate readily within homogenous rocks as well as between various rock layer (Goldberg, 1958).
Anthropogenic sources
Since ancient times, human activities have been adding large quantities of soluble salts to agricultural lands. In some areas, irrigation with unsuitable water under the high evaporation condition of arid or semi-arid climates and the practice of improper agrotechnological procedures have resulted in deposition of large quantities of salts in upper soil layers.
For examples, irrigation with water from the Jordan river (approx. 500mg/litre soluble) at an annual rate of 1000 m3 water per 1000 m2 land, adds at least a net 500 gm of salts per square meter to the soil. Excess, irrigation also causes leaching of salts into low sites and eventually results in formation of salt marshes.
With development of industry and extensive use of fuel, i.e. since the second half of the nineteenth century, another source of soluble salts on land surface has been added.
For example, a fair sized crude oil refinery with a capacity of a million tons per annum, releases about 45,000 tons of sulfuric acid into the atmosphere, if the average content of sulfur in the fuel is only 1.5%. According to Loewingart (1958), even if only 10% of such quantity enters into the annual cycle of renewing groundwater, it means an addition of 4 mg sulfate per liter of rainfall each year for a country of the size of Israel.
Electric power plants, metal smelters and automobile fumes may also add considerable amounts of pollutants, mostly of SO2 to limited areas.
Cement factories also spread annually thousands of tons of salts in their surroundings. Although the major contribution of such factories is in the form of calcium carbonate and calciuim hydroxide, the added salts are still of local importance. Urban use of water also results in increase of salinity. Only approximately 2/3rds of the continents are drained to the sea. The remaining areas e.g. deserts, which lack runoff or groundwater flow, are drained into maritime terminal drainage basin. Such systems contribute the source for formation of most terrestrial salines.
Salt cycles in nature
In nature, salts undergo numerous cycles of distribution, transport, and precipitation before accumulation occurs at certain sites.
5 major salt cycles, each having separate and different patterns, were recognized by Kovda (1961):
- Continental salt cycles
- Marine salt cycles
- Delta salt cycles
- Artesian cycles
- Anthropogenic salt cycles
1. Continental salt cycles: In this cycle, movements and accumulation of chloride, sulfate, and carbonate salts occurs in arid regions. Only a small amount of runoff or percolation of water occurs. Only a small portion of such salts reaches the ocean. Two types of sub-cycles are found:
- Primary salt cycles: Movement of salts originated by weathering or from salt-containing primary rocks.
- Secondary salt cycles: Movement of salts which originated by weathering of sedimentary rocks.
2. Marine salt cycles: Movement and accumulation of salts near sea coasts. Such salts return eventually into the oceans.
3. Delta salt cycle: Precipitation and accumulation of salts in deltas of large rivers.
4. Artesian salt cycles: Accumulation of salts from underground saline water sources on the land surface. Such cycles are long term ones. Salts which take part in such cycles are usually fossil.
5. Anthropogenic salt cycles: Movement and accumulation of salts produced and released by human activity.
Classification of saline habitats
On the basis of their specific nature and localities, saline habitats or salines are usually classified into a few types. One of the first modern classification of saline habitats was proposed by Stocker (1928). The classification is as follows:
1. Aquatic-haline
2. Terrestro-haline
- Hygrohaline
- Mesohaline
- Xerohaline
3. Aero-haline
- Habitats affected by salt spray (maritime)
- Habitats affected by salt dust (salt deserts).
This classification is based on the sources of salts and on the plant organs which they affect. Plants are divided according to their respective habitats and later subdivided according to their water relations. In all cases, salinity is defined as the soil salt content above 0.5% NaCl calculated on the soil dry weight basis.
Marine saline habitats
Walter (1968) classified saline habitats into few classes:
- Oligo haline 500-5000 mg/L
- Mesohaline 5000-18000 mg/L
- Polyhaline 18000-30000 mg/l
- Euhaline 30000 mg/L and above
The first 3 groups are commonly designated as brackish water.
Coastal saline habitats
Salines which are formed on coasts and are directly affected by the proximity of the sea are included in this group. Salt composition of such salines closely resembles that of seawater, although their salt content varies greatly with specific local conditions.
Coastal salines are formed all over the world under semi-arid and arid regions as well as under maritime temperate climates. Coastal salines can be classified into the following formations:
- Salt water swamps
- Salt marshes
- Salt bogs

1. Salt water swamps: A swamp in the temperate or subtropical regions is covered with woody plant community and occurs in an area where the soil is saturated or covered with surface water during a good part of growth season. In the tropics, the vegetation of inundated swamps may be of grasses or Papyrus.
The most typical plants of mangrove swamps are Rhizophora sp., Avicennia sp., Sonneratia sp.
2. Salt marshes: A marsh is a grass community in a habitat where the soil is saturated or covered with surface water during part of the growth season. Marshes are formed in the brackish water with wet soil surface or with a cover of surface water. Marshes are typical of temperate regions and are mostly covered with grass community as Spartina alterniflora or Spartina-Distichlis-Juncus communities

3. Salt bogs: Peat forming swamps are often called ‘bogs’ (or peaty bogs). These are usually saline with an abundant shrub vegetation that grows in habitats that receive only limited amounts of mineral sediments.
References
- Biology of Halophytes by Yoav Waisel.
 Plantlet The Blogging Platform of Department of Botany, University of Dhaka
Plantlet The Blogging Platform of Department of Botany, University of Dhaka






Really interesting seeming. Nice to read.
Thank you Bari. You have always been kind and active.
i want to know how does this 3d thing work.
Will get with an update soon.
You always add some new and interesting things to your articles that make them more attractive!
Thank you.
All the images are interesting specially the last one.
Thank you. I have used a gif here. Directly inserted it via link. You can try it in your articles.