In soil, there may be found 3 types of reactions. They are neutral, alkaline, or acidic reactions. The measure of the chemical reaction of soil(degree of alkalinity or acidity) is expressed by its pH value.
Soil pH
Soil pH is the negative logarithm of the concentration of hydrogen ion (expressed in moles per litre) present in the soil
- Sorenson (1909) first used the term ‘pH’.
- pH is the single electrochemical property of soil that influences all physical, chemical, and biological properties of soil.
Classification of Soil on the basis of pH value
The USDA classification of soils on the basis of soil pH is given below:
| Types of soils | pH value |
| Extremely acidic | Below 4.5 |
| Very strongly acidic | 4.5-5.0 |
| Strongly acidic | 5.1-5.5 |
| Medium acidic | 5.6-6.0 |
| Slightly acidic | 6.1-6.5 |
| Very slightly acidic | 6.6-6.9 |
| Neutral | 6.6-7.3 |
| Very mildly Alkaline | 7.1-7.3 |
| Mildly alkaline | 7.4-7.8 |
| Moderately alkaline | 7.9-8.4 |
| Strongly alkaline | 8.5-9.0 |
| Very strongly alkaline | 9.1-above |
- pH of agricultural soils: 5.0-8.5
- Best Range: 6.0-7.5
- Acid sulfate soils: pH below 4.0
- Present in Chittagong hill tracts, Chokoria, Teknaf, Cox’s Bazar
- Extremely acidic, so plants can’t grow
- Local name: Kosh Soil
- Due to extreme acidic conditions (presence of Aquaregia – HNO3.3HCl), soil color is darker.
- Saline soils: pH around 7.0
- KCl, NaCl remains present in large quantities
- As these salts have a pH of 7.0, the pH of these soils also remains around 7.0.
- Calcareous soils: pH 5-8.3
- Dominance of CaCO3
- Cultivation generally is not favoured at this pH
- Alkali Soils: pH more than 5
- If more than 15% Na+ remains present in the body of soil colloids, such soils are called alkali soils.
Soils having a pH of more than 7.0 are called alkaline soils.
Prove that the ionic product of water is 10-14
We know, Water is a poor conductor of electricity. In 107 (10 million) liters of pure water, only one molecule of water remains in dissociated state in following manner:
H2O ⇔ H+ + OH–
Now, according to the law of mass action, the equilibrium constant for this ionization can be written as,
Kw ⇔ [H+] * [OH-] / [H2O]
Here, [H2O] is close to unity.
So, Kw = [H+] * [OH–]
As in 107 litres of water, there remains = 1 mole H+
So, In 1 L of water, there remains =1/107 moles/L =10-7 moles/L
So, [H+] = 10-7 moles/L
Similarly, [OH–] = 10-7 moles/L
Kw = [10-7] * [10-7] = 10-14
So, Kw = [H+] . [OH–] = 10-14 [Proved]
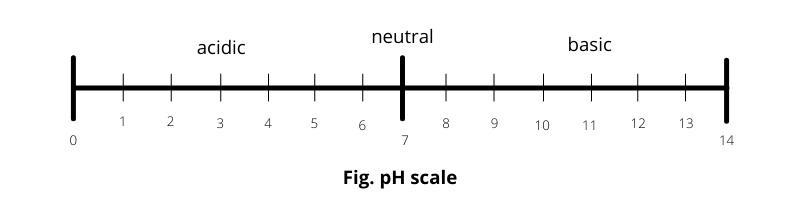
Explain: pH Scale runs between 0 and 14
We know, water is a poor conductor of electricity. In 107 (10 million) litres of pure water, only one molecule of water remains in dissociated state in following manner:
H2O ⇔ H+ + OH–
Now, according to the law of mass action, the equilibrium constant for this ionization can be written as,
Kw ⇔ [H+] * [OH-] / [H2O]
Here, [H2O] is close to unity.
So, Kw = [H+] * [OH–]
As in 107 litres of water, there remains = 1 mole H+
So, In 1 L of water, there remains = 1/107 moles/L = 10-7 moles/L
So, [H+] = 10-7 moles/L
Similarly, [OH–] = 10-7 moles/L
Kw = [10-7] * [10-7] = 10-14
We know, HCl acid is a strong acid, and thus it can get completely ionized.
Now, if 1N HCl is added to 1L solution, then the concentration of H+ is 1 mole/L
So, [H+] = 1 = 100
Therefore, pH = -log100 = 0
Similarly, for the strong base NaOH, when 1N NaOH is added to a 1L solution,
[OH–] = 1 mole/L
Since, [H+] * [OH–] = 10-14
Or, [H+] = 10-14/[OH–]
Or, [H+] = 10-14
Therefore, pH = -log10-14 = 14
Now, if we add further acid or base, these will remain in an undissociated state and thus will not affect the pH scale.
For this reason, the pH scale varies from 0 to 14.
Mathematical Problems
Q.1. Calculate the pH and OH– concentration of a solution containing H+ of 10-4 moles/L .
Solution: We know,
pH = -log[H+] = -log10-4 = 4 (Ans)
Again, ionic product of water,
[H+] × [OH–] = 10-14
Therefore, [OH–] = (10-14)/10-4 = 10-10 moles/L (Ans)
Q-2.The ionic product of Fe(OH)3 = 1.1 × 10-36 . Find the concentration of Fe3+ ion at pH 7 and pH 6 .
Solution: Here, ionic product, [Fe3+] × [OH–]3 = 1.1 10-36
Now, at pH 7, [OH–] is 10-7 mole/L
Therefore, [Fe3+] = ( 1.1 × 10-36 ) / [OH–]3 = ( 1.1 10-36 ) / (10-7)3 = 1.1 × 10-15 mole/L (Ans)
Similarly, at pH 6, [OH–] is 10-8 mole/L
Therefore, [Fe3+] = (1.1 10-36 ) / [OH–]3 = ( 1.1 × 10-36 ) / (10-8)3 = 1.1 × 10-12 mole/L (Ans)
Sources of Hydrogen Ions in Soil
1. Organic Matter
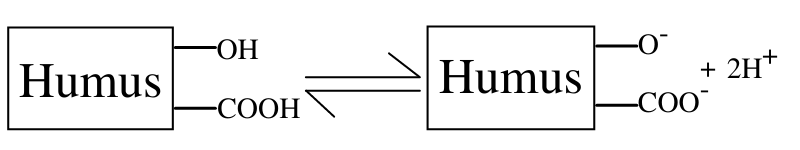
1.1 Production of Carboxylic (-COOH) and phenolic (R-OH) acid groups
The humic and fulvic acids of humus are sources of hydrogen ions because they contain carboxylic(-COOH) and phenolic (R-OH) groups, and thus dissociation reactions involving humus act as an important source of H+ ions in soil.
1.2 Production of CO2 during decomposition of organic matter
Perhaps, the most important contributor of soil acidity is the formation of and subsequent dissociation of H+ ions from carbonic acid. This weak acid is formed when CO2 from soil air dissolves in water. Root respiration and decomposition of organic matter by microorganisms produce high levels of CO2 in soil air.
CO2 + H2O ⇔ H+ + HCO3– ⇔ H+ + CO32-
1.2 Humus reacts with Fe and Al ions and forms complexes which may undergo hydrolysis to give H+ ions
2. Aluminosilicate clays
The absorbed Al is in equilibrium with Al3+ ions in the soil solution and Al3+ ions contribute to soil acidity through their tendency to hydrolyze.

The aluminium ions then get hydrolyzed to aluminium hydroxy ions.
Al3+ + H2O ⇔ Al(OH)2+ + H+
Al(OH)2+ + H2O ⇔ Al(OH)2+ + H+
Al(OH)2+ + H2O ⇔ Al(OH)3 + H+
The H+ ions thus released lower the pH of soil solution and are major sources of H+ ions in most acid soils.
3. Hydrous Oxides
Principally, these are oxides of iron and aluminium. They may occur as-
- Amorphous particles of colloidal and dimensions,
- Crystalline colloidal materials such as Gibbsite,
- Coatings on other mineral particles,
- Interlayers between crystal lattice structures.
Under acid conditions, these oxides may be brought into solution and undergo stepwise hydrolysis with the release of H+ ions.
Fe3+ +HOH ⇔ Fe(OH)2+ + H+
Fe(OH)2+ +HOH ⇔ Fe(OH)2+ + H+
Fe(OH)2+ + HOH ⇔ Fe(OH)3 + H+
4. Soluble salts
Salts may be either acidic, neutral, or basic originating from fertilizers, mineralization of organic matter, or weathering of minerals. These salts release H+ ions upon dissolution and thus act their source.
5. Sulfur Oxidation
The decomposition of plant residues commonly involves the oxidation of organic – S to yield H2SO4.
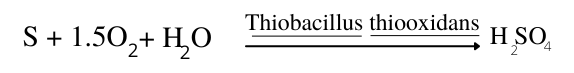
Another important source of this strong acid is the oxidation of reduced sulfur in minerals such as pyrite in acid-sulfate soils.
FeS2 + 3 O2 + H2O ⇔ FeSO4 + 2H+ + SO42-
These reactions are responsible for producing large amounts of acidity in certain soils in which reduced sulfur is plentiful and oxygen levels are increased by drainage or excavation.
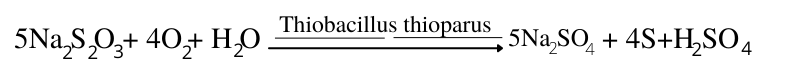
6. Plant uptake of cations
Plants maintain a balance between the positive and negative charges on the ions they take up from the soil solution. For every positive charge taken as a cation, a root maintains the charge balance either by taking up a negative charge as an anion or by exuding a positive charge as a different cation. When they take up far more of certain cations (e.g., K+, NH4+, Ca2+) than anions (e.g., NO3–, SO42- ), plants usually exude H+ ions into the soil solution to maintain charge balance.
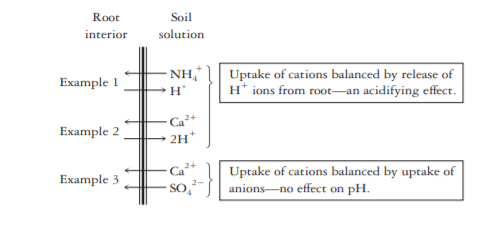
7. Acids in Precipitation
Precipitation (rain, snow, fog, dust, etc.) contains various acids that contribute H+ ions to the soil receiving precipitation. As raindrops fall through unpolluted air, they dissolve CO2 and form enough carbonic acid to lower the pH of soil water from 7.0 to about 5.6.
Varying amounts of sulfuric and nitric acids are formed in precipitation from certain nitrogen and
Source. Added to the author
sulfur gases are produced by lightning, volcanic, eruption, forest fires, and the combustion of fossil fuels.
SO2 +H2O ⇒ H2SO3 ⇒ (Oxidation) H2SO4
3NO2 + H2O ⇒ 2HNO3 + NO
Unlike carbonic acid, these strong acids completely dissociate to form H+, SO42-, and NO3– ions.
H2SO4 ⇔ 2H+ +SO42-
HNO3 ⇔ H+ + NO3–
Sources of Hydroxyl Ions in Soil
1. Removal of H+ and Al3+ ions by Ca2+, Mg2+, K+ (base-forming cations)
When adsorbed H+ and Al3+ ions are replaced from acid soils by cations such as Ca2+, Mg2+, and K+ the hydrogen ion concentration in the soil solution decreases, and these base-forming cations become the sources of OH ions merely by replacing the adsorbed hydrogen. Hydrolysis of base-forming cations thus can be a source of OH ions.
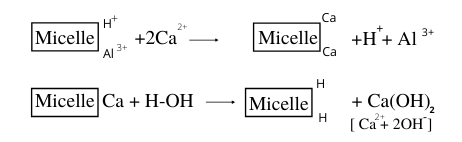
2. Alkaline reaction results from hydrolysis of colloids
The metallic cations, such as Ca2+, Mg2+, and K+ also have a more direct effect on the hydroxyl (OH–) ion concentration of the soil solution. A definite alkaline reaction results from the hydrolysis of colloids saturated with these cations. Under natural conditions, the reactions to furnish H+ ions and OH– ions to the soil solution occur at the same time. The pH of the soil solution will depend upon the relative amounts of adsorbed metallic cations compared to H+ and Al3+ ions. That is, where the effects of H+ and Al3+ ions are dominant, acidity results, and somewhere excess bases yield alkalinity.
3. Lowering of pH induces activation of basic groups by induced acceptance of proton which in turn induces adsorbed OH– ions and its subsequent by salt.
R-OH + H-OH ⇔ R-OH2 + OH–
R-OH2+OH– + SO42- ⇔ (R-OH2)2SO4 + 2OH–
4. Ligand exchange
In ligand exchange, orthophosphate ion (H2PO4–) replaces the hydroxyl group (-OH) from the surface of oxide minerals and releases OH– ions in soil solution.
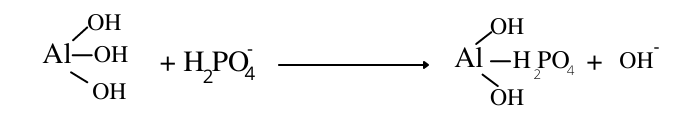
5. Hydrolysis of salts of strong bases and weak acids:
Hydrolysis of salts of strong bases and weak acids produces bases, which in turn give OH– ions in soil solution. It generally occurs in arid region soils.
Na2CO3 + H-OH ⇔ NaOH (Strong alkali; soluble in water; increases pH) + CO2 + H2O
6. Mineral Weathering
The weathering of many primary minerals affects alkalinity. This is the result of the consumption of H+ and the production of OH–. For example, the hydrolysis of anorthite, (calcium feldspar), generates a moderately strong base:
3CaAl2Si2O8 (anorthite) + 6H2O = 2HAl4Si6O10(OH)2 (aluminosilicate) + 3Ca(OH)2
In the reaction, an aluminosilicate clay mineral was formed together with the moderately strong base, calcium hydroxide. The net effect is basic. The generalized weathering reaction, in which M means metal ions such as Ca, Mg, K, or Na, is
M-silicate mineral + H2O = H-silicate mineral + M+ + OH–
As long as a soil system remains calcareous, hydrolysis of carbonate dominates the system and results in a pH that ranges from 7.5 to 8.3 or more. Mineral weathering is the very little amount under these cases, and the mineralogy and pH of the soils change minor, if at all, with time.
References & Other Links
- The Nature and Properties of Soils Fifteenth edition by Ray R. Weil | Nyle C. Brady
- Fundamentals of Soil Science by Henry D. Foth.
 Plantlet The Blogging Platform of Department of Botany, University of Dhaka
Plantlet The Blogging Platform of Department of Botany, University of Dhaka






Nice to read. I collect a lot of infos about soil pH here. Thanks
That’s great. Thanks for your feedback