Isotonic, Hypotonic and Hypertonic solution
Case A
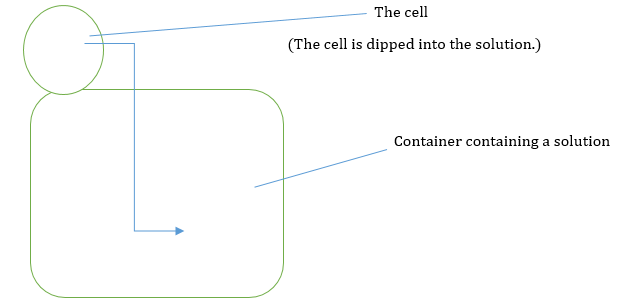
In the cell,
- Concentration = .1mMol L-1
- Ψp is negligible or 0
In the solution of the container,
- Concentration = .1mMol L-1
- Ψp = 0
In both cases, Ψw = Ψπ + Ψp = -CRT (Ψp is 0 or negligible)
Comment: There will be no exchange of water. The solution in the container is called isotonic solution.
Case B
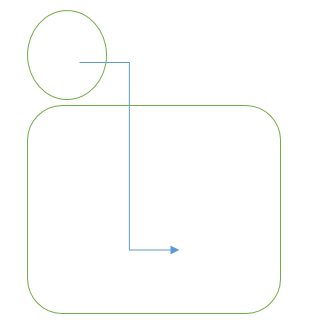
In the cell,
- Concentration = 5mMol L-1
- Ψw = -5RT
Best safe and secure cloud storage with password protection
Get Envato Elements, Prime Video, Hotstar and Netflix For Free
Best Money Earning Website 100$ Day
#1 Top ranking article submission website
In the solution of the container,
- Concentration = 2mMolL-1
- Ψw = -2RT
Comment:
- Water moves from container to cell.
- The solution is called hypotonic solution. (Hypo means below)
Case C

In the cell,
- Concentration = 2mMol L-1
- Ψw = -2RT
In the solution of the container,
- Concentration = 5mMolL-1
- Ψw = -5RT
Comment:
- Water moves from cell sap to existing solution.
- The solution in the container is called hypertonic solution.
Case B and case C are two different cases of osmosis
- Case B is endo-osmosis.
- Case C is exo-osmosis.
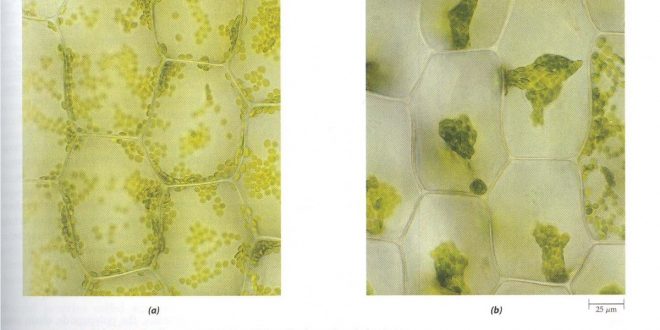
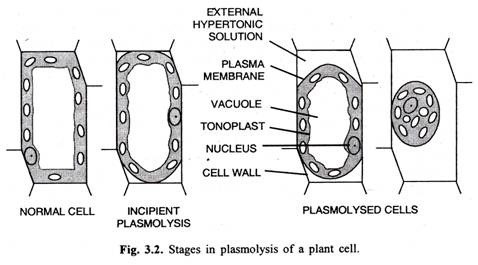
Plamolysis
The cell membrane of a cell shrinks after water comes out (about 90%) of the cell.
The contraction or shrinkage of the protoplasm away from the cell wall due to exo-osmosis when placed in a hypertonic solution is called plasmolysis and such a cell is called plasmolysed cell.
Now, after plasmolysis the cell of case C becomes like cell of case B.
So, endo-osmosis takes place. This is also called De-plasmolysis.
- The protoplasm forms a spherical form.
- Because of the permeable cell wall the space in between the cell wall and plasma membrane in plasmolysed cells is filled with outer hypertonic solution.
Importance of plasmolysis:
- Plants die as the salinity of the soil increases (Case C).
- Honey preparation.
- It indicates the semi-permeable nature of plasma membrane.
- The phenomenon is utilised in salting of meat and fishes and addition of concentrated sugar solution to jams and jellies to check the growth of fungi and bacteria which become plasmolysed in concentrated solution.
- It is also used in determining the O.P of the cell sap.
Imbibition
Imbibition is the absorption of water by dry hydrophilic colloidal substances in the form of protein, carbohydrates such as starch, cellulose, pectic substances etc. present both in living and dead cells of plants.
Dry pieces of wood or seeds if kept in water, they swell after a few moments. Here, the dry pieces are called imbibants and the process is called imbibition. Here, the imbibed substance is water.
Pressure caused by imbibition is called imbibition pressure and the pressure is not negligible.
Experiment: Prove that imbibition pressure is not negligible.
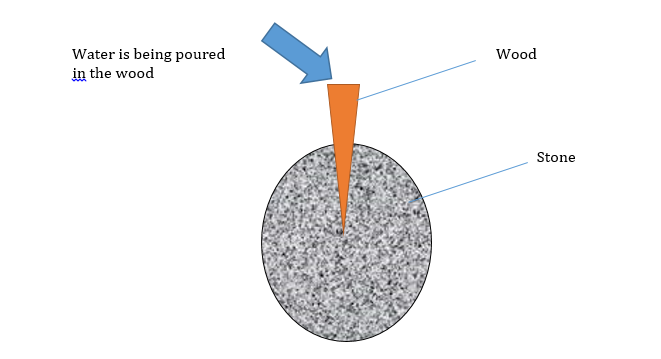
Result:
- The stone collapses after some period.
Importance
- This is the first step to take water by the cell walls of the root hairs and then the other three processes occur.
- Imbibition of water is very essential for dry seeds before they start germination.
 Plantlet The Blogging Platform of Department of Botany, University of Dhaka
Plantlet The Blogging Platform of Department of Botany, University of Dhaka