Chemistry is about combining different substances. Sometimes combining substances can cause a chemical reaction and bonding. This creates an entirely new substance called a compound. However, sometimes when there is no chemical reaction or bonding, it forms only a mixture. A mixture is made when two or more substances are combined, but not chemically. General properties of a mixture:
- We can easily separate the components of a mixture.
- The components each keep their original properties.
- The proportion of the components is variable.
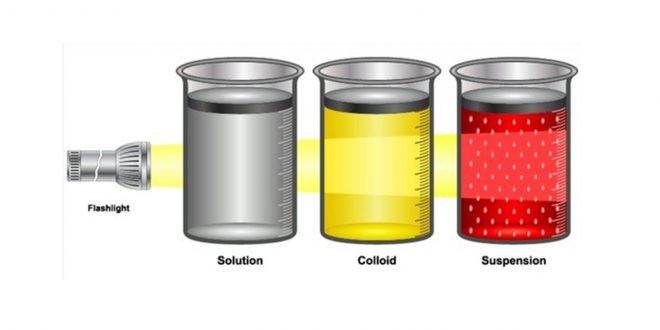
Solution, Suspension, and Colloid
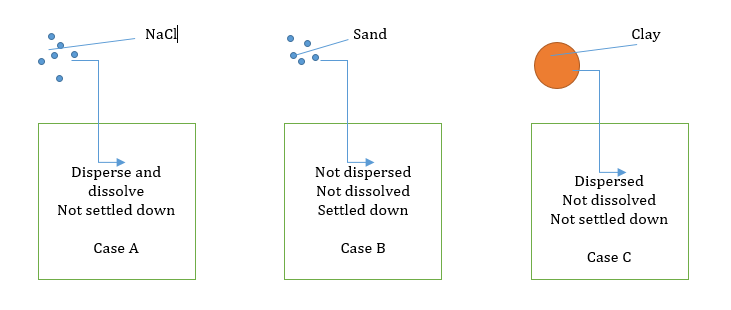
In case A (Solution)
- Particle size: 10-7 to 10-8
- Can’t be detected even under the ultra-microscope.
- This is called a solution.
- Solution system is stable because the molecules or ions do not settle down.
- The solvent can be solid or gaseous too.
- The solution can pass through a parchment membrane or collodion.
- A solution is a homogeneous mixture.
In case B (Suspension)
- Particle size: 10-3 to 10-4/5
- Due to the larger size of the particles, they are visible under a light microscope and in coarse suspension can be seen even with naked eyes.
- This is called a suspension.
- The suspension is an unstable system.
Best safe and secure cloud storage with password protection
Get Envato Elements, Prime Video, Hotstar and Netflix For Free
Best Money Earning Website 100$ Day
#1 Top ranking article submission website
In case C (Colloid)
- Particle size 10-6/5 to 10-7 cm or 1-200 nm.
- We can see clay particles by electron microscope.
- This is called a colloidal suspension or colloidal system.
- The colloid system is heterogeneous.
- The system is stable.
- Most colloidal particles can pass through filter paper, but they cannot pass through cellophane, as can truly solute particles.
- The colloidal system is of 8 types.
Three of them are:
- Solid + Liquid = Colloidal solution/sol (They have fluid like consistency)
- Solid + Gas = Tobacco + Smoke
- Liquid + Liquid = Milk
In type 1, solid (clay) is the dispersed phase or discontinuous phase and liquid (water) is the dispersed medium or continuous phase.
Origin of name
Scientist Graham in 1861 first used the term ‘colloid’ to describe glue-like preparation such as a solution of certain proteins and liquid preparation of vegetable gums. Gk. ‘kola’ means glue and ‘eidos’ means like.
Colloidal solution/Sol
A mixture of two different components, where the solid forms a dispersed phase and the liquid forms a dispersion medium, the solution is called colloidal solution or sol. Basically, solid+liquid is a colloidal solution or sol. E.g. Protoplasm.
But protoplasm isn’t a true solution. It is a complex colloidal solution. The consistency of protoplasm is both of a sol and gel type. The cell membrane seems to be more gel-like in nature. However, both these forms are not static but constantly changing.
The particles of the dispersed phase in colloidal solution are called colloidal particles or Sol particles or micelles. The size of the colloidal particles is in between the size of particles of true solution and suspension.
Colloidal dimension in liquid can be classified into two general classes.
- Lyophobic (Solvent hating): When the colloidal particles and the solvent repel each other.
- Lyophilic (Solvent loving): When the colloidal particles and the solvent attract each other.
If the solvent is water, the solution is either hydrophobic or hydrophilic.
Lipid head outside
Lipid tail inside
The starch solution is sticky.
Why do colloids not settle down?
Because the surrounding constantly strike them Also they are much smaller than water molecules. In rapid motion, they are small enough that the random velocities of the impacting water molecules do not average out. At a given moment there is a high probability that a colloidal particle is bombarded more strongly on one side than on the opposite side. When we observe colloidal particles in a light microscope by strong illumination on one side, they appear as points of light (Tyndall effect). They seem to dance around with many random hops per second. The largest (brightest) particles dance less than the smaller (dimmer) particles.
This is the Brownian movement. It is a beautiful, even spectacular confirmation of the kinetic theory. This erratic and continuous motion keeps colloids from settling.
With slightly larger particles, there is a much greater chance that the random bombardment on any side will approach an average value for the entire particles. In the contest between kinetic bombardment and gravity, gravity wins, so the particle settles.
Many particles in a cell, including the ribosomes and all the single protein molecules that are enzymes, are in the colloidal size range.
Because of the small size of the colloidal particles, their total surface in a given volume is relatively huge. The reactions of life occur on surfaces, and it is easy to see how relatively large surfaces can exist in a single cell.
The smaller the radius of curvature of the meniscus, the tighter the water is held to the colloidal or other hydrophilic surfaces by hydration, and the more negative the matric potential.
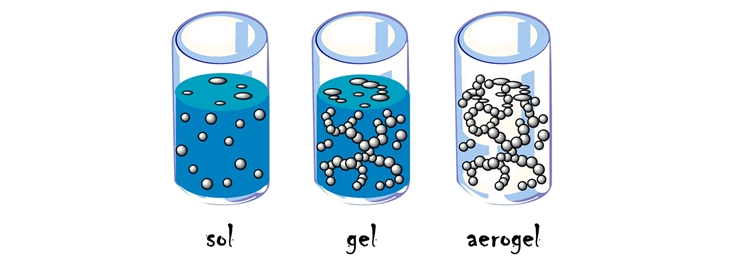
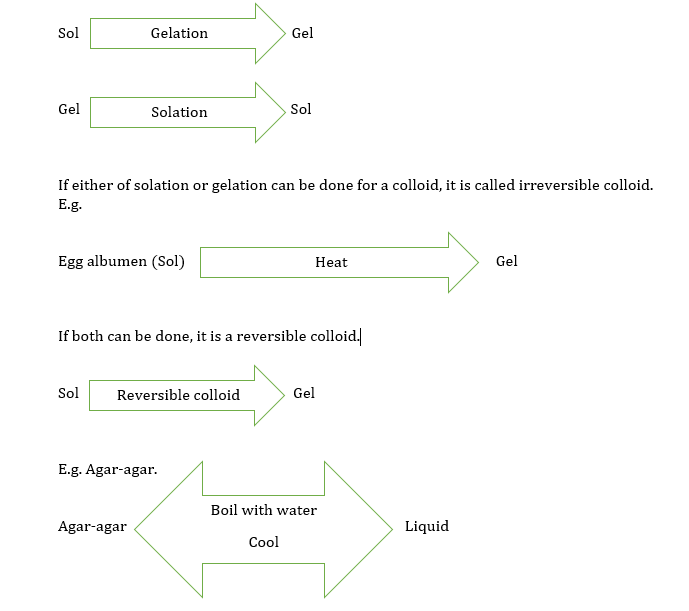
Gelation and solation
Under certain conditions, the sol may change into another form having a semi-solid consistency. We call it gel. And the name of the process is Gelation. During this process, the two phases of the sol become reversed in a gel.
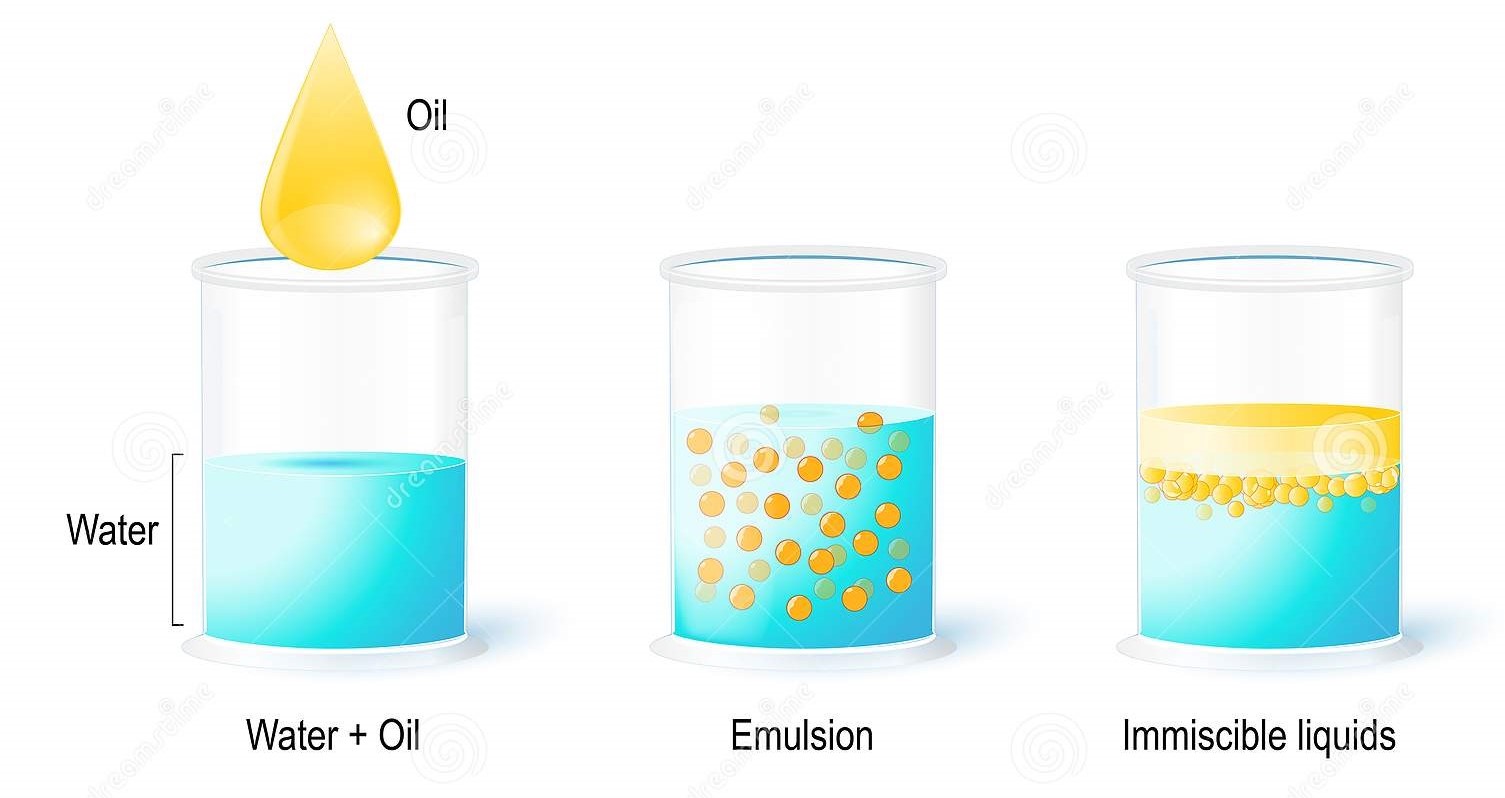
Emulsion
Emulsion is a kind of colloidal system.
Dispersion of small drops of one liquid in another liquid with which it is immiscible is called an emulsion. We can easily prepare it by mixing a few drops of oil into the water in a test tube and shaking it well. However, this emulsion will be unstable because after some time the tiny oil drops will coalesce together to form a separate layer. The dispersed phase is small in quantity. An emulsion is an unstable solution. We can shake it to make it unstable. We can also make emulsion into a stable mixture by adding the emulsifying agent.
While agitating butterfat in a container, we get butter up and water below.
Emulsifying agents
- Stops the function of surface tension by coating the particles.
- e.g. Milk, buffer fat, etc.
- Casein is an emulsifying agent.
- Heat can denature enzymes.
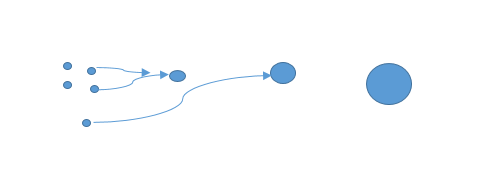
Figure: Emulsifying Particles Gathering Together.
Revised by
- Noushin Sharmili Suzana on 18 August 2021.
 Plantlet The Blogging Platform of Department of Botany, University of Dhaka
Plantlet The Blogging Platform of Department of Botany, University of Dhaka





Your article gave me a lot of inspiration, I hope you can explain your point of view in more detail, because I have some doubts, thank you.
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.