All of us might have heard about nicotine, cocaine and morphine. These are some bad world’s words that we always keep us away from. However, in biochemistry, a beautiful science where every natural compounds are discussed, these bad words along with some beauties have taken a very special sub-field known as alkaloids.
So first thing first….what is an alkaloid?
Definition
Any of various organic compounds normally with basic chemical properties and usually containing at least one nitrogen atom in a heterocyclic ring, occuring chiefly in many vascular plants and some fungi. Many alkaloids, such as nicotine, quinine, cocaine and morphine are known for their poisonous or medicinal attributes.
Discovery
In 1804, the German chemist Fredrich Serturner isolated from opium a ‘soporific principle’ which he called ‘Morphium’ in honour of Morphoeus, the Greek God of dreams. The term ‘Morphine’ was given by the French physicist Josph Gay-Lussac.
The name ‘alkaloids’ (German Alkaloide) was introduced in 1819 by thye German chemist Carl F W Meissner.
Characteristics
- Alkaloids are secondary metabolites.
- They are a group of naturally occuring chemical compounds that contain mostly basic nitrogen atoms which are derived from amino acids.
- Extremely heterogenous group.

Berberine. Source here - High molecular weight.
- Large compounds.
- All basic in reactions.
- Most of them are colorless (exception: Berberine is yellow), crystalline, non-volatile solids but some are liquids (e.g. coniine, nicotine) at ordinary temp..
- Usually bitter in taste.
- Insoluble or poorly soluble in water but soluble in most of the organic solvents.
- Physiologically active when eaten by animals.
- Often synthesized in a particular plant organ but accumulate in another e.g. in tobacco, nicotine is synthesized in roots but is translocated to and stored in leaves.
- They have been isolated from the roots, leaves, barks of stems or seeds of the plants.
- Stored in the vacuoles or in the cytoplasm in the solid form.
e.g.
- morphine from opium poppy.
- nicotine from tobacco.
- quinine from cinchona
- atropine from nightshaqde.
- colchichine from meadow saffron.
Good to know
- Secondary metabolites: Secondary metabolites or natural products can be defined as a heterogeneous group of natural metabolic products that are not essential for vegetative growth of the producing organisms, but they are considered differentiation compounds conferring adaptive roles, for example, by functioning as defense compounds or signaling molecules in ecological interactions, symbiosis, metal transport, competition, and so on. (1)
Distribution
- Do not appear in young cells till vacuolation takes place.
- Are hardly found in dead tissues.
- Occur only in the parenchyma.
- Accumulate chiefly in 4 kinds of tissues- a. actively growing tissues, b. epidermal and hypodermal cells, c. vascular sheaths and d. latex vessels.
- Are scattered almost in every group of plants (except algae), are very rare in gymnosperms. The dicotyledonous plants are genuine producers of alkaloids.
- More than 10000 different alkaloids have been discovered i.e. species from over 300 plant families.
- Are commonly common in Magnoliaceae, Solanaceae, Papaveraceae (all of its members contain alkaloids), Leguminosae, Ranunculaceae, Rubiaceae, Apocynaceae etc.
Classification
Alkaloids are often divided into 3 major groups.
- Protoalkaloids
- True alkaloids
- Pseudo alkaloids
Protoalkaloids
- Contain simple structured nitrogen and originate from amino acids.
- Do not contain any heterocyclic ring.
- Examples include: Herdenine, ephedrine, mescaline and adrenaline.
True alkaloids
- Contain nitrogen in the heterocycle.
- Usually originate from amino acids.
- Have a bitter taste and appear as white salt.
- On the basis of ring system present in their molecules, true alkaloids are further classified into 4 groups.
- Alkaloids derived from aliphatic Amino Acids (AAs).
- Alkaloids derived from aromatic AAs.
- Alkaloids derived from AA tryptophan.
- Pure alkaloids.
- Alkaloids derived from aliphatic Amino Acids
- These alkaloids are again divided into 3 groups.
- Quinolizidine alkaloids: Lupinine, Sparteine, Luparine etc.
- Nicotiana alkaloids: Nicotine, Nornicotine, Nicotinic acids etc.
- Tropane alkaloids: Hyoscyamine, Cocaine, Atropine etc.
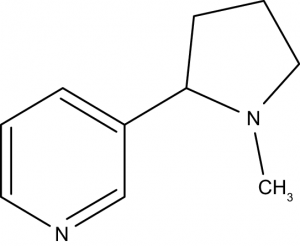
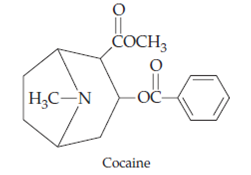
2. Alkaloids derived from aromatic Amino Acids
- These alkaloids are again divided into 4 groups.
- Amaryllidaceae alkaloids: Belladine.
- Colchichine: Colchicine.
- Betaxanthins and betacyanins: Indicaxanthin, Betanidine.
- Isoquinoline alkaloids: Papaverine, Morphine.
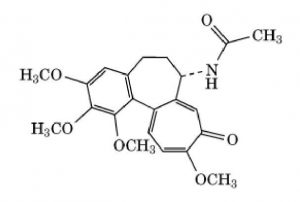
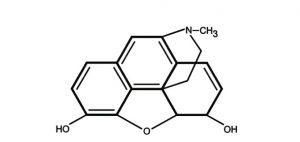
3. Alkaloids derived from Amino Acid tryptophan
- These are indole alkaloids.
- Each of them has an indole group which are derived from amino acid tryptophan.
- E.g. Reserpine, quinine etc..
4. Purine alkaloids
- They have a purine ring.
- E.g. Caffeine, theophylline etc..
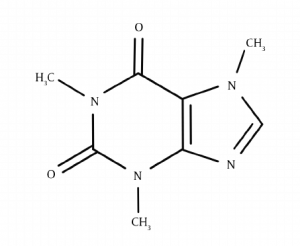
Pseudoalkaloids
- Alkaloid like compounds that do not originate from amino acids.
This group includes terpene like and steroid like alkaloids. - Examples: coniine*, demissidine etc.
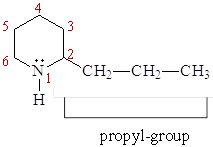
Good to know
- Coniine is a poisonous alkaloid. It is usually isolated from poison hemlock (Conium maculatum) and some other plant species. In 399 BC, Socrates, when he was sentenced to death, chose to die by drinking a coniine-containing mixture of poison hemlock.
- Terpene
- Steroid
Physiological role of alkaloids
- Provide protection against predator.
- Act as N reserve.
- Act as growth regulator (especially as germination inhibitors).
- Some alkaloids are used as insecticides.
- Are used in medicines usually in the form of salts.
| Alkaloids | Action |
| Vinblastine | Antitumer |
| Vinplastine | Antitumer |
| Codeine | Cough medicine |
| Cocaine | Anesthetic |
| Morphine | Pain reducer |
| Tubocurarine | Muscle relaxant |
- Used as psychoactive substance.
- Cocaine and cathinone are stimulants of the central nervous system. Many of indole alkaloids (e.g. psilocybin and ibogaine) have hallucinogenic effect. Morphine and codeine are strong narcotic pain killer.
- Nearly all of the alkaloids are poisonous in large amounts.
Source:
- https://www.tankonyvtar.hu/
- https://www.researchgate.net/
- https://www.chegg.com/
Best safe and secure cloud storage with password protection
Get Envato Elements, Prime Video, Hotstar and Netflix For Free
 Plantlet The Blogging Platform of Department of Botany, University of Dhaka
Plantlet The Blogging Platform of Department of Botany, University of Dhaka





