Concept of water potential
- Free energy per mole is the chemical potential.
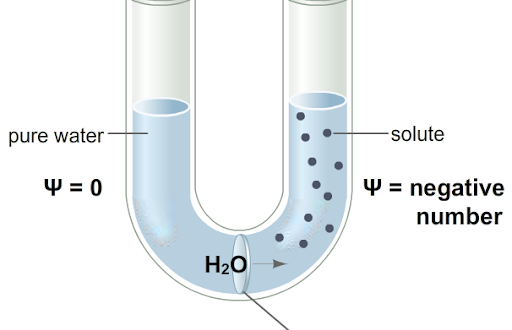
- The water potential is the chemical potential of a water solution in a system minus the chemical potential of pure water at atmospheric pressure and at the same temperature.
- A system’s water potential expresses its ability to do work compared with the ability in a comparable quantity of pure water at atmospheric pressure and at the same temperature.
According to thermodynamic laws every component of a system possesses free energy capable of doing work under constant temperature conditions. Osmotic movement…….
Osmotic pressure (O.P) in a solution results due to the presence of solutes and the latter lower the water potential. Therefore, osmotic pressure is a quantitative index of the lowering of water potential in a solution and using thermodynamic terminology is called osmotic potential (Ψs). Osmotic pressure and osmotic potential values are equal but while the former has positive sign, the latter carries a negative sign (If OP = 20 atms then, Ψs is -20 atms).
In an open osmotic system, the water potential and the osmotic potential values are numerically similar and also have same sign i.e. negative (similar will be the case of plasmolysed cells). On the other hand, in a closed osmotic system e.g. plant cells a pressure is imposed on water which increases the water potential. In plants, the pressure is called turgor pressure. This is the actual pressure with positive sign and ranges between zero and numerical osmotic potential value. The potential created by such pressures is called hydrostatic pressure or pressure potential (Ψp). Thus, Ψw = Ψp + Ψπ.
Best safe and secure cloud storage with password protection
Get Envato Elements, Prime Video, Hotstar and Netflix For Free
Best Money Earning Website 100$ Day
#1 Top ranking article submission website
| Ψw = Ψs (As Ψp= nil) | Ψw Lowest | In plasmolysed or flaccid cell. |
| Ψw = Ψp + Ψπ | Ψw Higher | In partially turgid cell. |
| Ψw = 0 (As Ψp numerically equals Ψs but both having opposite signs) | Ψw Highest | In fully turgid cell. |
If for example,
…………..
Vant Hoff’s equation,
Ψs = – CiRT where,
- C = Concentration of solution expressed as molality (i.e. moles of solute per kg of water Unit: mol kg-1).
- i = The activity coefficient. It is a constant that accounts for ionization of the solute and/or other deviations from perfect solutions.
For non-electrolytes/non-ionised molecules such as sucrose, glucose, sorbitol, mannitol or PEG (Polyethylene glycol), it is 1.
But for electrolytes such as NaCl it varies with their concentration and is invariably less than one. - Gas constant (.00831 kg MPa mol-1K-1 or .0831 kg bars mol-1K-1 or .08025 kg atm mol-1K-1)
- T = Absolute temperature (K).
- Total Ψs for a complex solution such as cell sap is the sum of all osmotic potentials caused by all solutes. It is called Osmolality.
Problem
For 1.0 molal glucose solution at 30 degree celcius and 0 degree celcius, find the osmotic potentials and comment on the relation between temp. and solute potential and find the direction of flow of glucose. (Salisbury pg: 49)
Solution:
Good to know
- 1 bar = 106 dynes/sq. cm
- 1 bar = .987 atm
- 1 MPa = 10 bar
- 1 MPa = 9.87 atm
- 1 MPa = 1 KJ Kg-1 (Pressure and energy relation)
Factors creating water potential gradients
Concentration
Water is almost incompressible. So, its concentration remains nearly constant. Thus a model based strictly upon water concentration will not explain diffusion of water in plants. Indeed differences in water potential can readily be ignored.
Temperature
- The cooler gas diffuse into the warmer gas as the cooler gas is the more concentrated gas when the pressures are equal, and the concentration difference is more important to diffusion than the slightly higher velocities of the molecules in the warmer gas. So temperature effects on diffusion are complex.
Actually temp differences are usually ignored in soil-plant-air continuum as we assume temp. to be constant throughout the system and the environment. But this isn’t applicable for plants like growing in tundra region.
- The temperature effect is much greater than the solute or pressure effects.
Pressure
Increasing the pressure increases the free energy and hence the chemical potential in a system.
If pressure is applied to the solution on one side of the membrane but not to the other, the water potential of the pressurized side will be increased; there will then be a net movement of water molecules by diffusion through the membrane and into the compartment of lower pressure.
This is extremely important in studying plants because the contents in most plant cells are under pressure compared with their surroundings, and because fluids in the xylem can be under tension (negative pressure).
Effects of solutes on solvent chemical potential or just solutes
- Solute particles decreases the free energy and chemical potential of the solvent molecules.
- Solute potential is high on the solution side and infinitely low on the pure water side.
- Solutes can also move across membranes against chemical potential gradient, but metabolic energy must be used to do so.
Matrix or adsorptive surface
- Many charged surfaces such as those of clay particles in soil, proteins or cell wall polysaccharides have a great affinity for water molecules.
- Even surfaces that have no net charge also bind water.
- A material with surfaces that bind water is called a matrix.
- Binding is a spontaneous process that releases free energy (ΔG is negative).
- The process of water being adsorbed by a matrix is called hydration or imbibition. It is primarily responsible for the first phase of water uptake by a seed prior to germination.
Good to know
- Pressure increases free energies and chemical potential.
- Solute particles and matric surfaces decrease free energies and chemical potential.
- It is the plant cell membrane that makes osmosis possible, but it is the cell wall that provides the rigidity to allow a buildup in pressure. Animal cells do not have walls, so when pressure build up in them, they often burst, as happens when red blood cells are placed in water.
- Turgid cells provide much of the rigidity of nonwoody plant parts.
- Adding solute decreases the water potential to some level below zero, and adding pressure raises this toward zero.
- If pure water is on one side of the membrane, pressure on the other side will increase until the water potential of the solution is equal to zero; i.e. equal to the water potential on the other side. So in equilibrium: ΔΨ = Ψ1 – Ψ2 = 0.
- The main goal is to reach equilibrium i.e. same water potential on both side (not necessarily need to be zero).
- Osmotic potential is zero in pure water.
- Water potential can be negative, zero, or positive. In pure water under some external pressure above atmospheric, water potential is positive. But note that in land plants, Ψ is never positive. This is because Ψs caused by solutes in cells is always negative, and matric forces also lower Ψ below zero. Pressure in cells can raise Ψ close to zero but never positive. Thus xylem sap, although it is nearly pure water and could have a positive Ψ if it were under pressure, stays under tension (negative pressure) as it remains at least close to equilibrium with the living tissues and their negative water potentials. But a submerged plant may have a positive Ψ.
- In a solution under some pressure other than atmospheric, water potential may be negative (osmotic potential is more negative than pressure is positive), zero (pressure equals osmotic potential but is opposite in sign), or positive (pressure is more positive than osmotic potential is negative).
- Osmotic potential of a solution is negative because the solvent water in that solution can do less work than pure water.
- As pressure on the solution increases, the solvent’s ability to do work also increases.
- The work is performed by movement of pure water into the solution.
- Leaf wilts when the turgor pressure is zero.
 Plantlet The Blogging Platform of Department of Botany, University of Dhaka
Plantlet The Blogging Platform of Department of Botany, University of Dhaka





The point of view of your article has taught me a lot, and I already know how to improve the paper, thank you.