The sulfur cycle is the collection of processes by which sulfur moves between rocks, waterways and living systems. Such biogeochemical cycles are important in geology because they affect many minerals. Biochemical cycles are also important for life because sulfur is an essential element, being a constituent of many proteins and cofactors, and sulfur compounds can be used as oxidants or reductants in microbial respiration.[1]
Source of Sulfur
Oxidation states of Sulfur
Sulfur, a reactive element with stable valence states from -2 to +6 is among the most abundant elements in the earth’s crust. It has four main oxidation states in nature, which are -2, +2, +4, and +6. The common sulfur species of each oxidation state are listed as follows:
- S2-: H2S, FeS, FeS2, CuS
- S0: native, or elemental, sulfur
- S2+: SO
- S4+: SO2, sulfite (SO32-)
- S6+: SO42- (H2SO4, CaSO4), SF6
In living organisms, sulfur occurs mainly as sulfhydryl (-SH) groups in amino acids (e.g. Cysteine) and their polymers. Plants, algae and many heterotrophic microorganisms assimilate sulfur in the form of sulfate for incorporation into cysteine, methionine and coenzymes in the form of sulfhydryl (-SH) groups, sulfate needs to be reduced to sulfide level by assimilatory sulfate reduction.
In the marine environment, a major decomposition product of organosulfur is dimethyl sulfide (DMS, (CH3)2S). This product originates from dimethylsulfoniopropionate (DMSP), a major metabolite of marine algae that may have a role in osmoregulation.
Good to know
- Oxidation is the loss of electrons or addition of oxygen or removal of hydrogen.
- Reduction is the gain of electrons or removal of oxygen or addition of hydrogen.
Best safe and secure cloud storage with password protection
Get Envato Elements, Prime Video, Hotstar and Netflix For Free
Best Money Earning Website 100$ Day
#1 Top ranking article submission website
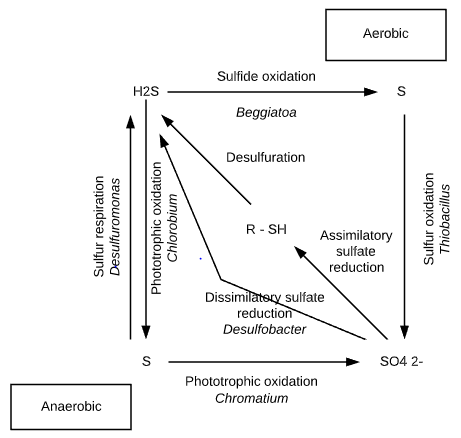
Oxidation sulfur transformation
1. Oxidation of reduced S compounds
In the presence of oxygen,
reduced sulfur compounds (e.g. H2S) are capable of supporting chemolithotrophic microbial metabolism. Beggiates, Thioploca, Thiothrix and the thermophilic Thermothrix are filamentous, microaerophilic bacteria capable of oxidizing H2S.
H2S + 1.5 O2 = S + H2O
Sulfur globules are deposited within the cells of bacteria. In the absence of H2S, these globules are slowly oxidized to sulfate. Read more.
2. Oxidation of elemental S and inorganic S compounds
Some other members of the genus Thiobacillus species produce sulfate from the oxidation of elemental sulfur and other inorganic sulfur compounds.
S + 1.5 O2 + H2O = H2SO4
These Thiobacillus species are acidophilic, grow well at pH 2-3, and are obligate chemolithotrophs, obtaining their energy exclusively from the oxidation of inorganic sulfur and their carbon from the reduction of carbon dioxide. Most Thiobacillus species are obligate aerobes requiring oxygen for the oxidation of inorganic sulfur compounds.
3. Oxidation of inorganic S compounds (using nitrate as electron acceptors)
Thiobacillus denitrificans however can utilize nitrate ions as terminal electron acceptors in the oxidation of inorganic sulfur compounds.
3 S + 4NO3– = 3 SO4 2- + 2 N2
4.
Sulfur oxidation produces a substantial amount of strong mineral acid. Within soils, this can lead to solubilization and mobilization of phosphorus and other mineral nutrients with a generally beneficial effect on both microorganisms and plants. The activity of Thiobacillus thiooxidans may be used for adjusting soil pH. T. thiooxidans and T. ferrooxidans are used in microbial mining operations.
5. Phototrophic oxidation of H2S
Hydrogen sulfide (H2S) is also subject to phototrophic oxidation in aerobic environments. Photosynthetic sulfur bacteria, the Chromatiaceae, Ectothiorhodospiraceae, and Chlorobiaceae, are capable of photo reducing CO2.
CO2 + H2S = (CH2O) + S
CO2 + H20 = (CH2O) + O2 (Photosynthesis)
The formula (CH2O) symbolizes photosynthate.
Reductive sulfur transformation
1. Reduction of elemental S in anaerobic respiration
Elemental sulfur can be used in the respiratory process. Desulfuromonas acetoxidans grow on acetate anaerobically. It uses elemental sulfur instead of oxygen in its energy-producing metabolism.
CH3COOH + 2 H2O + 4 S = 2 CO2 + 4 H2S
Desulfuromonas occurs in anaerobic sediments rich in sulfide and elemental sulfur. It also lives syntrophically with the phototrophic green sulfur bacteria that photooxidize H2S to S and excrete elemental sulfur extracellularly.
2. Reduction of S by Hydrogen gas
From submarine hydrothermal vent environments, extremely thermophilic anaerobic archaea were isolated that are capable of sulfur respiration with hydrogen gas. Several species of Thermoproteus, Pyrobaculum and Pyrodictium were described to carry out this chemolithotrophic reaction. Pyrodictium occultum is the most thermophilic member of the group, having an optimal growth temperature of 110 C. It is probably an advantage for these extreme thermophiles that sulfur is present in hydrothermal vent regions in a molten state, whereas at room temperature it is a hydrophobic solid. Sulfur respiration occurs with molecular hydrogen.
H2 + S = H2S
3. Reduction of sulfates
The reduction of sulfate results in the production of hydrogen sulfide. When obligately anaerobic bacteria carry out dissimilatory sulfate reduction, they are referred to as sulfate reducers or sulfidogens.
Desulfobacter, Desulfobulbus, Desulfococcus etc. are the sulfate reducers.
5 H2 + 2 SO3 2- = 2 H2S + 2 H20 + 2 OH–
Sulfate reduction contributes to the atmospheric cycling of sulfur. Until recently it was believed that most of the biogenic sulfur transfer to the atmosphere.
Revised by
- Md. Siddiq Hasan on 24 March, 2021
 Plantlet The Blogging Platform of Department of Botany, University of Dhaka
Plantlet The Blogging Platform of Department of Botany, University of Dhaka