Properties of water
- Most of the unique properties of water are ascribed to the H bond formed between polar water molecules.
- The two H atoms are angled at 105° in a water molecule (?).
1. Surface tension
Surface tension results due to forces of attraction existing between the molecules of a liquid at the open boundary surface of that liquid and is measured by the force per unit length (newton/metre) acting in the surface in the right angle to any line drawn in the surface.
Cohesion among water molecules results for surface tension. The molecules at the surface of the liquid are continuously being pulled into the liquid by the cohesive (H bonds) forces, whereas those in the vapour phase are too few and too distant to exert any force on the molecules at the surface. As a result, a drop of water acts as if it were covered by a tight elastic skin.
Best safe and secure cloud storage with password protection
Get Envato Elements, Prime Video, Hotstar and Netflix For Free
Best Money Earning Website 100$ Day
#1 Top ranking article submission website
- Surface tension is responsible for transpiration pull that facilitates ascend of sap in higher plants.
- Under normal pressure, surface tension prevents the passage of air bubbles through the minute pores and pits in cell walls. The water surface of bubbles cannot deform enough to pass through the small openings.
2. Cohesion and adhesion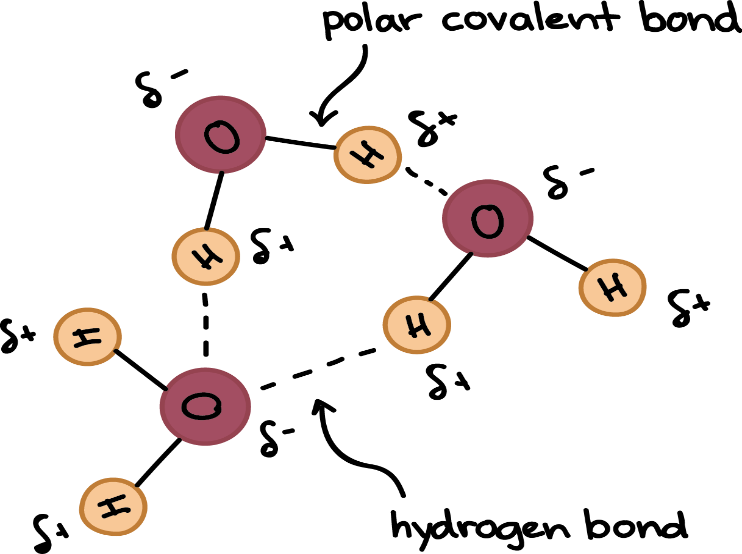
Mutual force of attraction between like molecules such as in water (due to H bonds) is called as cohesion. Cohesion confers upon water an unusually high tensile strength, which is the ability to resist stretching (tension) without breaking. In a thin confined column of water, such as that in the xylem elements of stem, this tensile strength can reach such high values that water is pulled to the top of tall trees without breaking.
On the other hand, attraction of water to a solid phase such as cell wall or glass surface is called as adhesion. Adhesive property is found in water due to its polar nature. It attracts other unlike but highly polar substances such as proteins and cell wall polysaccharides.
- Cohesive and adhesive properties of water are of great significance in ascend of sap in plants.
- e. the water molecules are sticky (?).
3. Evaporation process
- Liquid state to gaseous state.
- Unit to measure evaporation is vapour pressure gradient.
- One needs temperature and humidity to calculate vapour pressure.
Mechanism of water movement inside plants
The movement of materials into and out of the cells in plants takes place in solution or gaseous form.
There are three mechanisms that govern water movement inside the plant
- Mass flow
- Diffusion
- Osmosis
Mass flow
Mass flow is the movement of water from a high concentrated region to a less concentrated region.
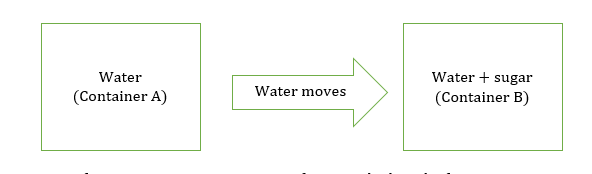
Water moves from container A to B as conc. of water is high in the first container. In container B, the conc. of water is less as some of its molecules are bounded with sugar molecules.
- Ψ is the potential of water to move or simply as water potential.
- For pure water, potential of water, Ψpure water = 0
- It indicates that anything dissolved in water will lower the value of Ψ.
- If Ψ is negative, then something (e.g. Na+, salt or sugar etc.) is dissolved in water.
- Unit of Ψ is bar, atmosphere or pascal.
- Ψπ or, s is solute potential.
Diffusion
- Concentration is the amount of substance or number of particles per unit volume.
- A gradient occurs when a parameter such as concentration changes gradually from one volume of space to another. A temperature gradient is easy to visualize; it could be expressed as change in temperature with change in distance: ΔT Δx-1.
- Liquid water is virtually incompressible.
- The concentration of water remains nearly constant at 55.2 to 55.5 M (mol L-1).
- Addition of many substances, such as various sugars or salts, causes the water volume to expand, so the water concentration is diminished slightly.
Addition of certain other substances actually causes the water volume to shrink slightly, increasing the water concentration. In no cases can theses changes in water concentration account in a quantitative way for the observed diffusion of water.
- Solute diffuses in response to differences in solute chemical potential. Similarly, water diffuses in response to differences in water potential.
- Driving force for diffusion is gradient. There are five factors that most commonly produce water potential gradients in soil-plant-air continuum.
They are: Concentration, temperature, pressure, effects of solutes on solvent chemical potential and matrix.
Overall movement of any particles or molecules in a system from a region of higher concentration to a region of lower concentration until its distribution is in equilibrium is called diffusion.
Equilibrium distribution doesn’t mean that the system has stopped working. Rather, it means that the equilibrium is dynamic. That is the passing of molecules occurs at same rate at the same probability.
- The rate of diffusion of gases is faster than liquids or solutes.
- The diffusing particles have a certain pressure called as the diffusion pressure which is directly proportional to the number or concentration of the diffusing particles.
- Diffusion process is spontaneous; free energy is released to the surroundings and the system’s free energy decreases. This releases energy has the potential to do work, such as osmotically lifting water upward in the stems in the phenomenon known as root pressure.
- Equilibrium is reached when the change in free energy (ΔG) or the water potential difference (ΔΨ) is equal to zero. At this point, entropy for the system and its surrounding will be at maximum but entropy change (ΔS) will be zero.
- Water vapour molecules diffuse about 1.6 times as fast as CO2 molecules (because of their lower molecular weight) and that the atmosphere normally contains 10 to 100 times more water vapour than CO2.
Direction of diffusion
- Higher concentration of solution to lower concentration of solution.
- Higher diffusion pressure to lower diffusion pressure i.e. along the concentration gradient.
- A diffusing solute tends to move from regions of high chemical potential (free energy per mole) to regions of low chemical potential.
The rate of diffusion increases if,
- The dimension of pressure gradient is steeper.
- The temperature is increased.
- The density of the diffusing particles is lesser. (?)
- The medium through which diffusion occurs is less concentrated.
Importance
……………………….
Osmosis/Osmotic diffusion
When the diffusing substance is water through a semi-permeable membrane.
Plant allows only one liquid inside it and that is water.
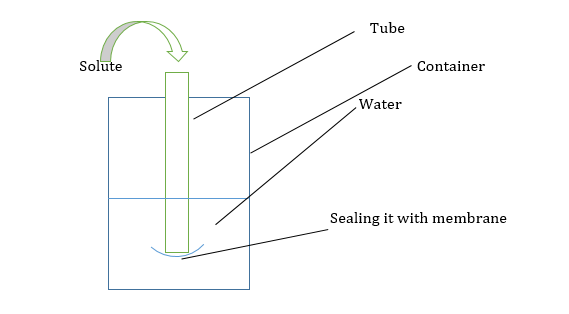
What is the direction of flow of water?
Water will flow from beaker to tube. As the Ψ in the beaker is 0 and in the tube is negative.
- Osmotic pressure is measured in terms of atmosphere.
- Osmotic pressure is directly proportional to the concentration of dissolved solutes in the solution. More concentrated solution has higher osmotic pressure.
- Osmotic pressure of solution is always higher than its pure solvent.
- Osmotic pressure does not increase by the addition of insoluble solute in the solution.
- Osmotic diffusion doesn’t take place in isotonic solutions.
- Osmosis is special type of diffusion.
Direction
- Less concentration of solution to high concentration of solution till both the solutions attain equal concentration.
- More concentrated area of water to less concentrated area of water.
- Solution of lower osmotic pressure (less concentrated or hypotonic) to the solution of higher osmotic pressure (more concentrated or hypertonic).
Significance of osmosis in plants
Simple posture of water relation
Water molecules possess a certain amount of kinetic energy. The greater the concentration of water in a system the greater is It’s kinetic energy or water potential. If two system containing water are in connect, movement of water molecules occur from the system with higher energy to the system with lower energy.
If a certain amount of solute is added to pure water,the concentration of water decreases and thus the water potential decreases.The amount by which the water potential decreases is called solute potential which is always negative and the value of solute potential decrease with an increase in the amount of the dissolved solutes.The value of water potential increases when a pressure kore than atmospheric pressure os applied to pure water.when water enters a plant call by diffusion and exerts a pressure on the walls of the cell,the cell is termed as turgid.This increase the pressure potentia. This value is usually positive.
So,water potential is the sum of solute potential and pressure potential.
Ψtotal =Ψs+Ψp
Where,Ψs=solute potential and Ψp=pressure potential.
What will happen of water movement between value of Ψ =-2 & Ψ=-40? Suppose, a pot is full of water where water molecules are present as H+ and OH– ions.
If we add a small amount of salt with Na+ and Cl– ions, salts positive ion then surround by waters negative ion.so,free ion of water will decrease and also Ψ value will be decrease. Suppose It’s decrease value is Ψ =-40.
Now another pot with no mixture of salt or any other particles rich in water ion.here Ψ value remains same or high(supposing its Ψ=-2) than the pot with salt mixture. If we make connect between these two pot then water movement will occur from pure water pot to pot with salt mixure.
Water movement will be from Ψ=-2 to Ψ=-40
Water always moves high to low water potential.
Revised by
- Ismayara Akter Liza added a significant portion of the article on 13 August, 2020.
 Plantlet The Blogging Platform of Department of Botany, University of Dhaka
Plantlet The Blogging Platform of Department of Botany, University of Dhaka






nice article. thank for sharing with us.